The Drug Administration of Vietnam ( Ministry of Health ) has officially licensed the circulation of the Russian-made drug Pembroria for cancer treatment.
Professor Le Van Quang, Director of K Hospital (Hanoi), said that the unit will soon use this drug to treat patients. According to him, Pembroria is not yet covered by health insurance.
Currently, Pembroria medicine produced by Limited Liability Company "PK-137" (Russia) costs about 18 million VND/bottle. Patients usually use 2 bottles for 1 treatment course; lasting 12-24 courses until no longer responding to the medicine, then stop. Each month, patients will be treated once.
According to Dr. Nguyen Hong Vu - who used to work at the City of Hope Research Institute, California, USA, Pembroria is an anti-cancer drug with the active ingredient Pembrolizumab, 100mg/4 mL, produced by Limited Liability Company "PK‑137".
The active ingredient Pembrolizumab, also found in the popular product Keytruda, is a humanized monoclonal antibody that targets PD‑1, a mechanism that helps “wake up” the patient’s immune system to attack cancer cells. The drug was developed from mice carrying human genes to create antibodies, then industrially produced on the CHO cell line.
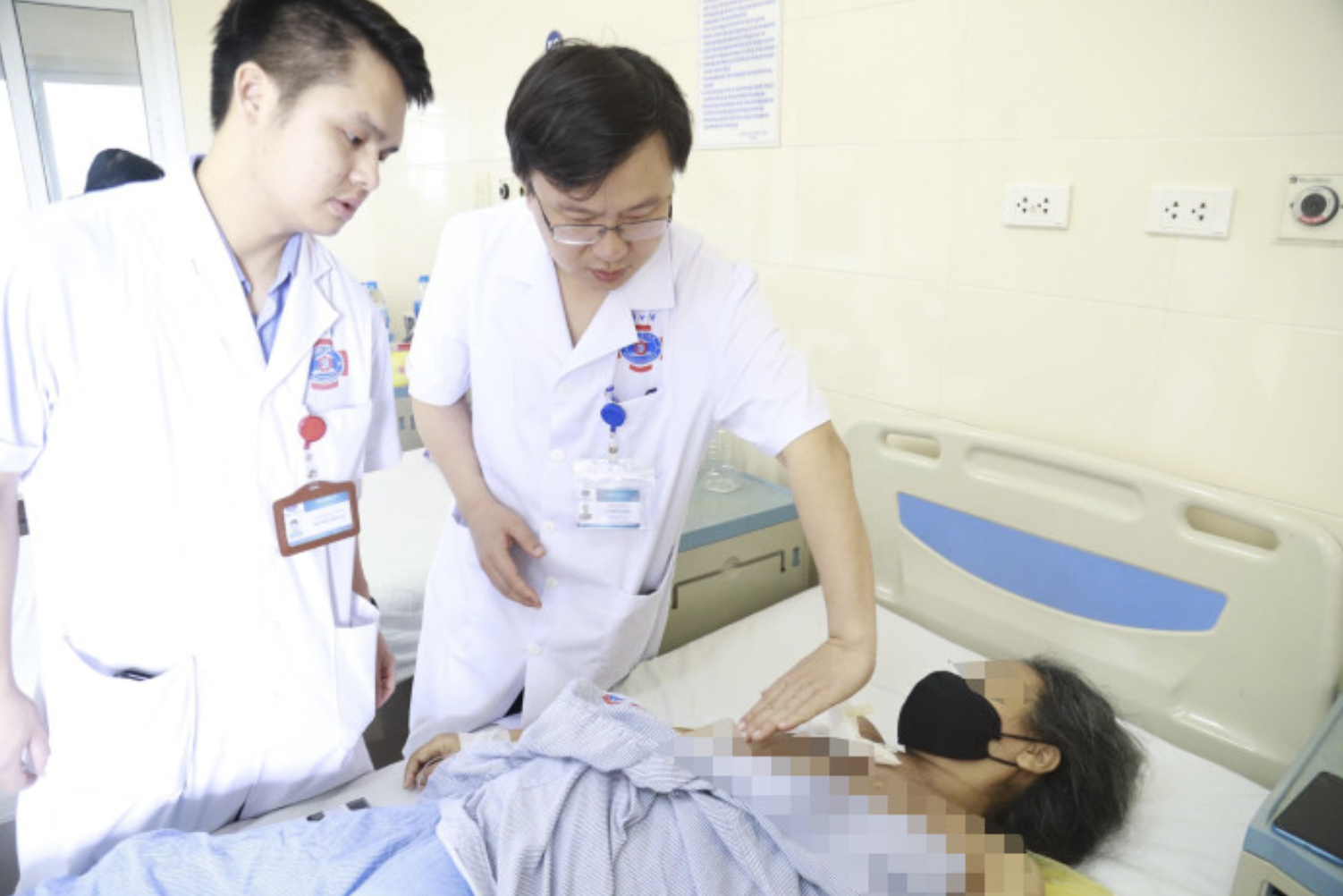
Cancer patients receiving treatment at K Hospital. Photo: Manh Tran.
Keytruda has undergone preclinical and phase I, II, and III clinical trials, and was first approved by the US Food and Drug Administration in 2014 for the treatment of malignant melanoma, and has since expanded to more than 15 other cancer indications.
Regarding Pembroria, Dr. Vu said that there is not enough internationally published clinical data to confirm the complete equivalence of Pembroria with Keytruda.
In the latest decision, the Drug Administration of Vietnam requires that after being granted a drug circulation registration certificate, drug manufacturers must periodically update the progress of phase III clinical research on immunogenicity monitoring every 3 months and submit documents to change and update phase III immunogenicity monitoring data when the research period ends.
According to information from the drug registration agency, Pembrolizumab has more than 14 indications for different types of cancer (such as lung carcinoma, melanoma, colorectal cancer, cervical cancer, renal cell carcinoma, breast cancer...).
Vietnamnet.vn
Source: https://vietnamnet.vn/benh-vien-k-noi-ve-viec-dua-thuoc-chua-ung-thu-cua-nga-vao-dieu-tri-2462018.html




![[Photo] Deep sea sand deposits, ancient wooden ship An Bang faces the risk of being buried again](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1763033175715_ndo_br_thuyen-1-jpg.webp)

![[Photo] Special class in Tra Linh](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/14/1763078485441_ndo_br_lop-hoc-7-jpg.webp)






























































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)






Comment (0)