According to the Drug Administration, the drug Pembroria (Russian cancer treatment drug) has the main active ingredient Pembrolizumab, content 100mg/4ml, produced by Limited Liability Company "PK-137" (Russia), registered by Anabion Pharmaceutical Trading Ltd (United Arab Emirates).
Pembroria medicine is prepared in the form of a concentrated solution for infusion, with a shelf life of 24 months from the date of manufacture.
According to information from the drug registration agency, Pembrolizumab has more than 14 indications for different types of cancer such as: Lung carcinoma, melanoma, colorectal cancer, cervical cancer, renal cell carcinoma, breast cancer...
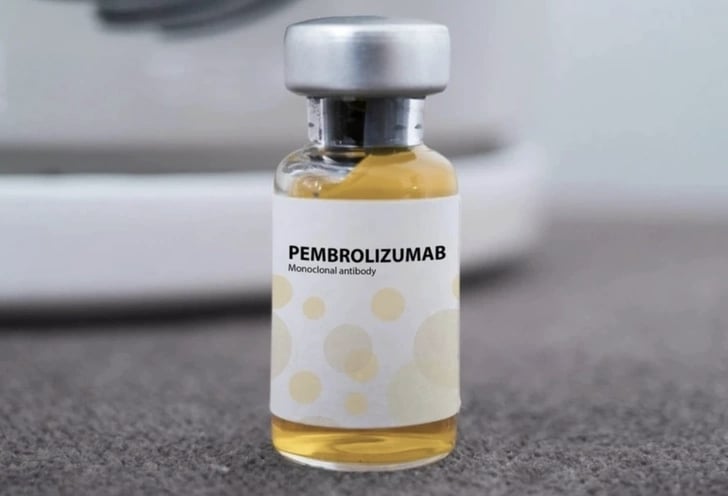
The product is licensed for circulation in Vietnam, helping patients have more access to advanced therapies.
In addition to cancer drugs, the Drug Administration also grants circulation licenses for vaccines and other medical biological products to treat stroke, lupus, osteoporosis, skin diseases, rheumatoid arthritis, ankylosing spondylitis, blood diseases, multiple sclerosis, etc.
The Drug Administration of Vietnam requests that drug manufacturing and registration establishments are responsible for producing and supplying drugs to Vietnam in accordance with the records and documents registered with the Ministry of Health .
Previously, at the bilateral talks in September, Minister of Health Dao Hong Lan and Minister of Health of the Russian Federation Mikhail Murashko discussed many directions of medical cooperation, notably the research and transfer of anti-cancer vaccines. This is an achievement announced by Russia in early September, expected to be revolutionary in cancer treatment.
The Vietnamese Ministry of Health is ready to cooperate extensively to access, share and transfer technology related to this vaccine, and expand cooperation in research, production and clinical trials in Vietnam.
Source: https://cand.com.vn/y-te/bo-y-te-cap-giay-dang-ky-luu-hanh-thuoc-chong-ung-thu-cua-nga-i787752/



![[Photo] Prime Minister Pham Minh Chinh receives Lao Minister of Labor and Welfare Phosay Sayasone](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/11/1762872028311_dsc-2246-jpg.webp)








![[Video] Many items are degraded as two large hospitals are slow to be put into use](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/12/1762906839099_dung00-29-15-18still010-jpg.webp)














































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)








Comment (0)