The Food Safety Department ( Ministry of Health ) has just issued decisions to revoke the validity of the Certificate of Acceptance of Product Declaration Registration for a series of functional foods of 4 companies.
Accordingly, 4 companies had their product registration certificates of some health protection foods revoked, including: Nano Pharma Pharmaceutical Joint Stock Company, Kingphar Vietnam Joint Stock Company, Nguyen Minh Pharmaceutical & Trading Company Limited, Vinh Thinh Vuong Production & Trading Company Limited.
Specifically, in Decision No. 1450/QD-ATTP, the Department of Food Safety decided to revoke the validity of the Certificate of Product Declaration Registration for the health protection food product Kingphar Super Kid , Certificate of Product Declaration Registration No. 13714/2019/DKSP issued on December 24, 2019.
Product announced by Vinh Thinh Vuong Production & Trading Company Limited, address: Binh Phu village, Yen Phu commune, Yen My district, Hung Yen province.
In Decision No. 313/QD-ATTP dated July 2, 2025, the Department of Food Safety decided to revoke the validity of the Certificate of Product Declaration Registration for the health protection food product Enterovina , Certificate of Product Declaration Registration No. 5803/2019/DKSP issued on May 23, 2019.
This product is announced by Nguyen Minh Trading & Pharmaceutical Company Limited, address: No. 45, Lane 79, Group 28, Quan Hoa Ward, Cau Giay District, Hanoi City.
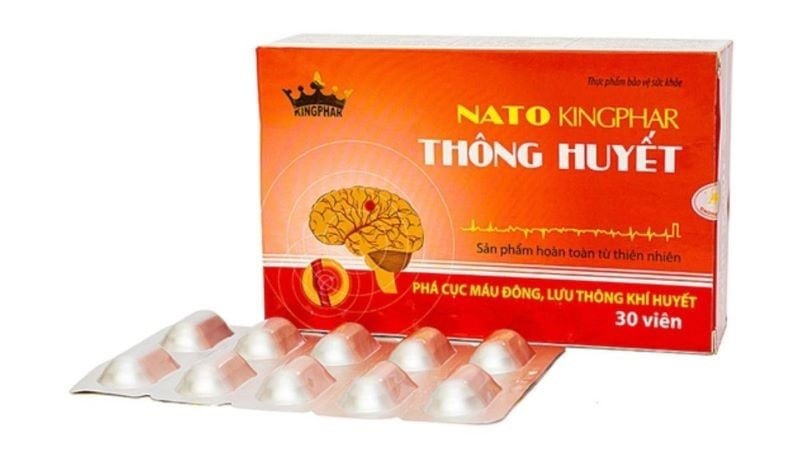
In Decision No. 314/QD-ATTP dated July 2, 2025, the Department of Food Safety decided to revoke the validity of the Certificate of Product Declaration Registration for 4 health protection food products announced by Kingphar Vietnam Joint Stock Company, address: Binh Phu village, Yen Phu commune, Yen My district, Hung Yen province.
These products include: Nato Thong Huyet-Kingphar; Menmoren ginkgo Plus; Digestive enzymes and Omega 3-6-9 Plus Q10 Kingphar.
In Decision No. 315/QD-ATTP dated July 2, 2025, the Department of Food Safety decided to revoke the validity of the Certificate of Product Declaration Registration for 2 health protection food products announced by Nano Pharma Pharmaceutical Joint Stock Company, located at 9 Nguyen Xien, Thanh Xuan Trung Ward, Thanh Xuan District, Hanoi City.
These two products include Nano IQ Mama Care (pills for pregnant women) and Nano IQ Aquamin F (calcium and vitamin supplement).
According to the Food Safety Department, the above decisions were made based on documents from companies requesting to withdraw documents related to the above products.
Source: https://nhandan.vn/bo-y-te-thu-hoi-hang-loat-giay-tiep-nhan-dang-ky-cong-bo-thuc-pham-chuc-nang-cua-4-cong-ty-post891511.html


























![[Photo] National Assembly Chairman Tran Thanh Man visits Vietnamese Heroic Mother Ta Thi Tran](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/7/20/765c0bd057dd44ad83ab89fe0255b783)





































































Comment (0)