Medical news September 29: More than 1,000 people received free cardiovascular examinations; Nam Ha Pharmaceutical was fined
Responding to World Heart Day, 1,000 people were screened for cardiovascular, kidney, and metabolic diseases at Bach Mai Hospital.
More than 1,000 people received free cardiovascular examinations
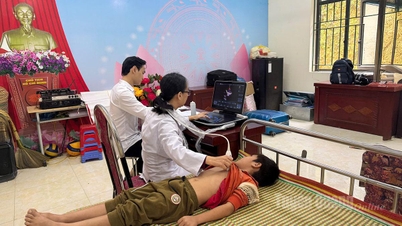
On September 29, 2024, at Bach Mai Hospital, the program to respond to World Heart Day took place - screening for cardiovascular, kidney, and metabolic diseases for 1,000 people.
 |
| In our country, each year about 200,000 people die from cardiovascular disease, accounting for 33% of deaths. |
This is a program summarizing a series of 3 events to celebrate World Heart Day, raising awareness of cardiovascular - kidney - metabolic diseases in 3 major cities across the country.
Cardiovascular disease is one of the leading causes of death in the world, as well as in Vietnam; accounting for about 17.9 million deaths each year, equivalent to 32% of total deaths in the world.
According to the World Health Organization in 2019, deaths due to cardiovascular diseases accounted for 39.5%, with cerebrovascular disease (55.4%) and coronary artery disease (32%) leading. These diseases cause burdens on health, quality of life and treatment costs for patients as well as caregivers.
In Vietnam, the trend of death due to cardiovascular disease is increasing, death due to cardiovascular disease is more than death due to cancer, COPD and diabetes combined.
In our country, every year about 200,000 people die from cardiovascular diseases, accounting for 33% of all deaths. Of which, deaths due to cerebrovascular diseases account for the largest proportion, with the mortality rate increasing from 127.3/100,000 people (in 2000) to 164.9/100,000 people today.
Besides cardiovascular disease, chronic kidney disease is a non-communicable disease commonly caused by diabetes and hypertension and is a serious public health problem.
Deaths due to chronic kidney disease accounted for 4.6% of deaths globally and were the 12th leading cause of death in 2017. In Vietnam, there are more than >8.7 million adults with chronic kidney disease, accounting for 12.8% of the population.
Vietnam currently has more than 400 hemodialysis units and provides dialysis services to about 30,000 patients with end-stage renal disease each year, but only meets 30% of the needs of patients needing dialysis nationwide.
According to the Vietnam Health Insurance report in 2022, the cost of paying for dialysis is currently at the top of the payment list, estimated at more than 4,000 billion.
Therefore, early diagnosis and treatment of chronic kidney disease, as well as slowing down the process of kidney function decline, renal replacement therapy will bring significant and long-term economic benefits while reducing the burden on the health sector.
However, the rate of missed diagnosis of chronic kidney disease is still very high, especially in the early stages due to atypical symptoms of the disease, only about 4.5-15.5% of patients with stage 3 chronic kidney disease are diagnosed, in which, the rate of missed diagnosis in some subjects at high risk of chronic kidney disease such as hypertension and diabetes is still high, respectively 68.4% and 51.7% (10).
Within the framework of the program, more than 1,000 people were examined and screened for free, bringing the total number of patients screened in September to more than 3,000.
The dedicated team of doctors and nurses conducted health checks and assessed potential risks related to cardiovascular and chronic kidney disease. Through this, people received practical advice on how to take care of their health, diet, and healthy lifestyle to prevent serious diseases.
Within the framework of the program, the Organizing Committee also presented 10 gifts to poor sick children, helping them have more joy and motivation in studying.
The Ministry of Health and the Vietnam Young Physicians Association have donated high-tech medical examination and treatment equipment to Bach Mai Hospital, including a mobile telehealth screen and an AI system that reads chest X-rays to detect tuberculosis and lung cancer.
This is an important step forward in improving the quality of local health services, and at the same time demonstrates the health sector's deep concern for public health. These devices not only help improve working conditions for medical staff but also bring many practical benefits to people in accessing advanced and modern health services.
The Ministry of Health requires ensuring the progress of ranking technical expertise of medical examination and treatment facilities.
The Ministry of Health has just sent a document to hospitals under the Ministry of Health, Departments of Health of provinces and centrally run cities, and health departments of ministries and branches on the implementation of technical and professional ranking of medical examination and treatment facilities.
Accordingly, the Ministry of Health requests the Directors of the Departments of Health of provinces and centrally-run cities, and the Heads of Health of Ministries and branches to study the document and classify medical examination and treatment facilities according to regulations;
Submit reports on the implemented work related to the technical expertise ranking of the Department of Health and implementation progress to the Ministry of Health before October 15, 2024;
Submit reports and results of the summary of the grading scoreboard according to Appendix V in Decree No. 96/2023/ND-CP of all affiliated hospitals in the management area and of private hospitals that have been granted operating licenses by the Ministry of Health, except for hospitals under the Ministry of Health, the Ministry of National Defense, and the Ministry of Public Security to the Ministry of Health (Department of Medical Examination and Treatment Management) before October 25, 2024.
The Ministry of Health requests that the Directors of hospitals under the Ministry of Health carry out self-assessment and complete the dossier requesting technical and professional classification, and send it to the Ministry of Health (Department of Medical Examination and Treatment Management) to carry out the classification according to regulations before October 15, 2024.
The Ministry of Health notes that all hospitals must self-assess and enter self-assessment results according to Appendix V in Decree No. 96/2023/ND-CP on the online software at chatluongbenhvien.vn before October 25, 2024.
According to the Ministry of Health, this is an important task. The Ministry of Health requests that the Heads of hospitals under the Ministry of Health, the Departments of Health of provinces and centrally run cities, and the Health of ministries and branches prioritize direction and urge implementation to ensure the roadmap prescribed in the Law on Medical Examination and Treatment No. 15/2023/QH15.
Nam Ha Pharmaceutical was fined for producing a batch of drugs that violated quality standards.
The Ministry of Health Inspectorate has just issued a decision to administratively sanction Nam Ha Pharmaceutical Joint Stock Company (located in Nam Dinh city, Nam Dinh province) for violating quality standards in a batch of Ubiheal 300 tablets commonly prescribed to treat nerve complications caused by diabetes.
This company produces Ubiheal 300 tablets, Registration number: VD-27692-17, batch number: 22103, production date: November 17, 2022, expiry date November 17, 2025, violating level 2 quality according to the provisions of law.
For this violation, the company was fined 70 million VND and forced to destroy the entire batch of Ubiheal 300 tablets above.
Ubiheal 300 of Nam Ha Pharmaceuticals has the main ingredient of thioctic acid 300mg. The drug is indicated for the treatment of sensory disorders and neurological complications caused by diabetes, such as atherosclerosis, macular degeneration, stroke, coronary artery disease, acute hepatitis, chronic hepatitis, liver toxicity...
Previously, in early September, the Drug Administration of Vietnam (Ministry of Health) issued an official dispatch announcing the recall of level 2 violations of the Ubiheal 300 tablet batch (Thioctic acid 300mg), Registration number: VD-27692-17, batch number: 22103 produced by Nam Ha Pharmaceutical Joint Stock Company because the drug sample did not meet quality standards in terms of solubility and quantification.
After this document, local health departments issued documents requesting the recall of drugs to ensure safety for users and prevent substandard drugs from circulating on the market. Medical facilities and pharmaceutical businesses are absolutely not allowed to distribute, trade, or use this batch of drugs.
Circular 11/2018/TT-BYT stipulates that level 2 violating drugs are drugs with evidence of not ensuring full treatment effectiveness or posing a risk of being unsafe for users but not to the extent of causing serious harm to health or affecting the life of the user.
The Ministry of Health stipulates that there are 24 cases considered to be level 2 violations of drugs, such as drugs that have been concluded by competent state agencies to not meet the requirements on treatment effectiveness; drugs produced from raw materials that do not meet quality standards;
Finished drugs are produced from expired raw materials or have been recalled by competent state agencies or do not have legal origins; drugs have a content exceeding the 5% limit compared to the limit specified in the registration dossier; drugs do not meet quality standards regarding bacterial contamination...
Source: https://baodautu.vn/tin-moi-y-te-ngay-299-hon-1000-nguoi-duoc-kham-tim-mach-mien-phi-duoc-pham-nam-ha-bi-xu-phat-d226139.html


![[Photo] Special class in Tra Linh](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/14/1763078485441_ndo_br_lop-hoc-7-jpg.webp)
![[Photo] Unique architecture of the deepest metro station in France](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/14/1763107592365_ga-sau-nhat-nuoc-phap-duy-1-6403-jpg.webp)
![[Photo] Unique art of painting Tuong masks](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/14/1763094089301_ndo_br_1-jpg.webp)





















![[Photo] Deep sea sand deposits, ancient wooden ship An Bang faces the risk of being buried again](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1763033175715_ndo_br_thuyen-1-jpg.webp)




































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)





Comment (0)