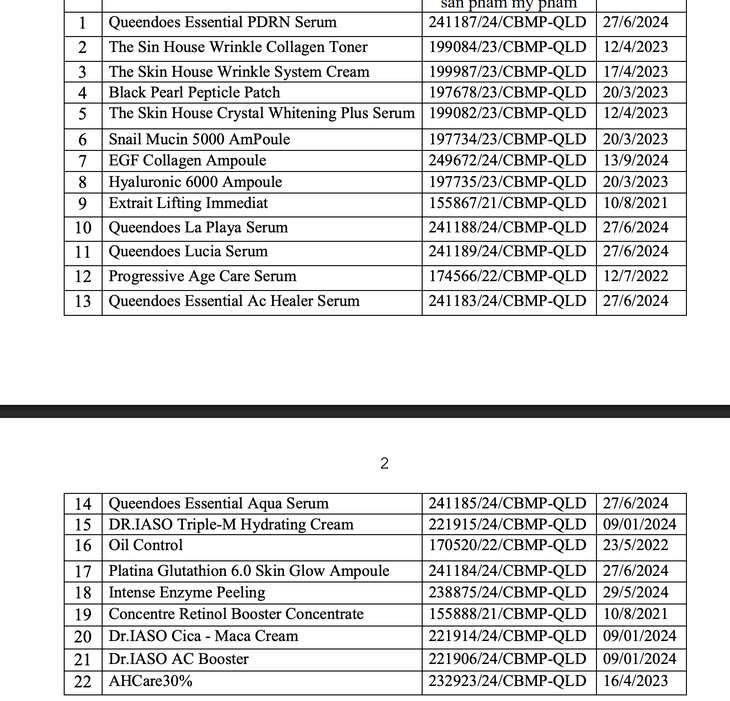
22 cosmetic products manufactured by Dai Cat A International Company Limited were recalled.
According to the Drug Administration, 22 cosmetic products brought to market by Dai Cat A International Company Limited (address: 43D/44 Ho Van Hue, Ho Chi Minh City) were suspended from circulation and recalled nationwide.
The reason for the recall is that the cosmetics company did not have or did not present the product information file (PIF) within the prescribed time limit when requested by the authorities for inspection.
For the same reason, the Drug Administration Department also decided to suspend circulation and recall 23 cosmetic products that Phat Anh Minh Company Limited was responsible for bringing to the market (address: No. 13D, Information Command's housing area, Hanoi City).
The Drug Administration of Vietnam requests the Department of Health of provinces and cities to widely notify business establishments, requesting them to immediately stop selling and using violating products, and organize recall and handling according to regulations.
The two companies must send recall notices to all distribution facilities, accept returned products and destroy all substandard cosmetics.
Previously, in June 2025, Dai Cat A International Company Limited was also suspended from circulation by the Drug Administration, recalling 20 types of cosmetics due to a series of violations related to circulating formulas that were not consistent with the published records, and labeling of uses that were not consistent with the published records.
23 cosmetic products made by Phat Anh Minh Company Limited were recalled.
In addition to the above two decisions, the Drug Administration also issued a document suspending circulation and recalling nationwide the batch of Panda baby Bach Lien Gel product, box of 1 tube of 30g (batch number: 310525P1NSX: 31-5-2025; HSD: 31-5-2028) of Nam Duoc Hai Long Joint Stock Company.
This product is recalled because the test sample does not meet the quality standards for microbial limits, does not ensure safety for users, and must be recalled nationwide.
The Drug Administration of Vietnam requests the Department of Health of provinces and centrally-run cities to immediately notify businesses and cosmetic users to stop selling and using suspended products.
Organize the recall, inspection, supervision and handling of organizations and individuals violating regulations. Require related businesses to strictly implement the recall notification, receive products from the market and destroy them according to regulations.
WILLOW
Source: https://tuoitre.vn/thu-hoi-46-loai-my-pham-tren-toan-quoc-20251114100350193.htm


![[Photo] Unique art of painting Tuong masks](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/14/1763094089301_ndo_br_1-jpg.webp)




![[Photo] Unique architecture of the deepest metro station in France](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/14/1763107592365_ga-sau-nhat-nuoc-phap-duy-1-6403-jpg.webp)

























![[Photo] Special class in Tra Linh](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/14/1763078485441_ndo_br_lop-hoc-7-jpg.webp)






































































Comment (0)