The above information was confirmed by the leader of the Drug Administration of Vietnam, Ministry of Health to Dan Tri reporter on the morning of November 11.
Specifically, according to Decision No. 628/QD-QLD dated October 31 issued by the Department of Drug Administration, Ministry of Health , there are 14 vaccines and biological products that have been granted a circulation registration certificate in Vietnam with a validity of 3 years - batch 57.
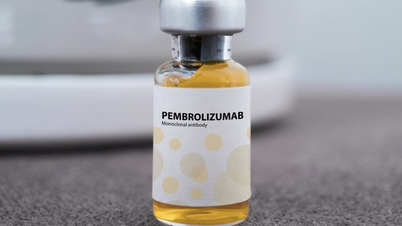
Among them, there is the drug Pembroria (main active ingredient is Pembrolizumab, content 100mg/4ml) produced by Limited Liability Company "PK-137" (Russia), registered by a facility in the United Arab Emirates.
Pembroria medicine is prepared in the form of a concentrated solution for infusion, with a shelf life of 24 months from the date of manufacture.

Pembroria medicine has the main active ingredient Pembrolizumab (Photo: incentra).
Notably, according to information from the drug registration agency, Pembrolizumab has more than 14 indications for different types of cancer (such as lung carcinoma, melanoma, colorectal cancer, cervical cancer, renal cell carcinoma, breast cancer...).
In the context of increasing demand for innovative cancer drugs and access to modern treatments for people, the licensing of Pembroria in Vietnam is of great significance.
The Russian Trade Representative Office in Vietnam said this is the result of continuous dialogue between specialized agencies of the two countries and the systematic work of this Office.
The update of the legal framework since the new Pharmaceutical Law came into effect has created favorable conditions to promote the import and circulation of Russian drugs, demonstrating the increasing level of trust and strategic partnership in the pharmaceutical sector between the two countries.
In the near future, according to experts, anti-cancer drugs, biotechnological drugs and essential drugs are most likely to be licensed.
Source: https://dantri.com.vn/suc-khoe/thuoc-chong-ung-thu-cua-nga-duoc-cap-dang-ky-luu-hanh-tai-viet-nam-20251111120511689.htm




![[Photo] Prime Minister Pham Minh Chinh receives Lao Minister of Labor and Welfare Phosay Sayasone](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/11/1762872028311_dsc-2246-jpg.webp)













![[Video] Many items are degraded as two large hospitals are slow to be put into use](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/12/1762906839099_dung00-29-15-18still010-jpg.webp)








































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)








Comment (0)