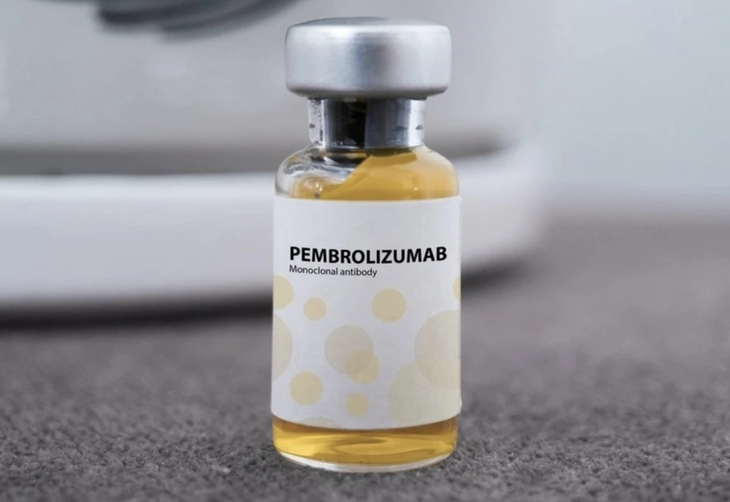
Pembroria medicine produced in Russia - Photo: Incentra
The Drug Administration of Vietnam, Ministry of Health has just granted circulation registration certificates in Vietnam for 14 vaccines and biological products. Among them is the drug Pembroria, the main active ingredient Pembrolizumab, content 100mg/4ml, produced by Limited Liability Company "PK-137" (Russia) and a facility in the United Arab Emirates registered for circulation in Vietnam.
Since yesterday, November 11, many people have been interested in this information because there is information that Pembroria has just entered Vietnam in the phase 3 clinical trial stage, not yet an official product, and its circulation is hasty. Another stream of information claims that this product is brand new and "brings hope to many patients", even calling it a "cancer vaccine".
Speaking to the press this morning, November 12, a representative of the Drug Administration Department said that both of the above information streams are inaccurate.
According to this person, before being granted a registration number for circulation in Vietnam, the product profile was evaluated by a pharmacology expert from Hanoi Medical University and confirmed to have fully met the requirements for effectiveness and immunogenicity similar to the original drug.
This product is in the form of a monoclonal antibody, has undergone clinical trials in phases 1, 2, 3 and the latest phase 3 ended in January 2024. However, when registering in Vietnam, the council required stricter requirements to continue to periodically evaluate every 3 months on immunogenicity, effectiveness, risk management...
"This requirement is higher than usual, it is not a product that has not completed clinical trials but has been registered for circulation as some people are misunderstanding. Another problem is that this is a treatment product, not a vaccine" - said a representative of the Drug Administration Department.
However, this monoclonal antibody is not a new invention, appearing for the first time in Vietnam, but Vietnam has circulated the same type of monoclonal antibody (generic drug) for many years before (since 2017). The Drug Administration has also licensed the circulation of 99 types of drugs and monoclonal antibodies for cancer treatment as of November 11, including Pembroria.
Like similar products, Pembroria is indicated for the treatment of melanoma, non-small cell lung carcinoma, classical Hodgkin lymphoma, urothelial carcinoma, esophageal carcinoma, colorectal cancer, cervical cancer, triple-negative breast cancer, gastric adenocarcinoma, cholangiocarcinoma...
Treatment should be prescribed and supervised by an experienced specialist. Depending on the circumstances, Pembroria can be used to treat adults or adolescents 12 years of age and older.
What you need to know about Pembroria monoclonal antibodies
The drug is manufactured by Biocad Company (Russia), registered for circulation in Vietnam by a company based in the UAE. The registration allows the drug to be imported, distributed and widely used like other drugs, because it does not belong to a special or restricted group of drugs.
Previously, in 2017, the drug Keytruda by MSD Group (USA), this is a generic drug with the active ingredient Pembrolizumab, was licensed in Vietnam.
Keytruda and Pembroria both contain the active ingredient Pembrolizumab, a monoclonal antibody that helps the body's immune system recognize and attack cancer cells.
Having more licensed drugs helps cancer patients in Vietnam have more options when using immunotherapy, in the context of increasing demand for cancer treatment. Especially in terms of product price, an expert said that the price of this product will certainly be lower than the original drug, although the owner of the original drug also has a roadmap to reduce the price of the product.
In Russia, Pembroria is approved from April 2022.
Source: https://tuoitre.vn/nga-cancer-treatment-drug-is-approved-or-is-it-just-a-sang-treatment-test-or-is-it-just-a-sang-test-in-viet-nam-20251112120926097.htm





































































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)








Comment (0)