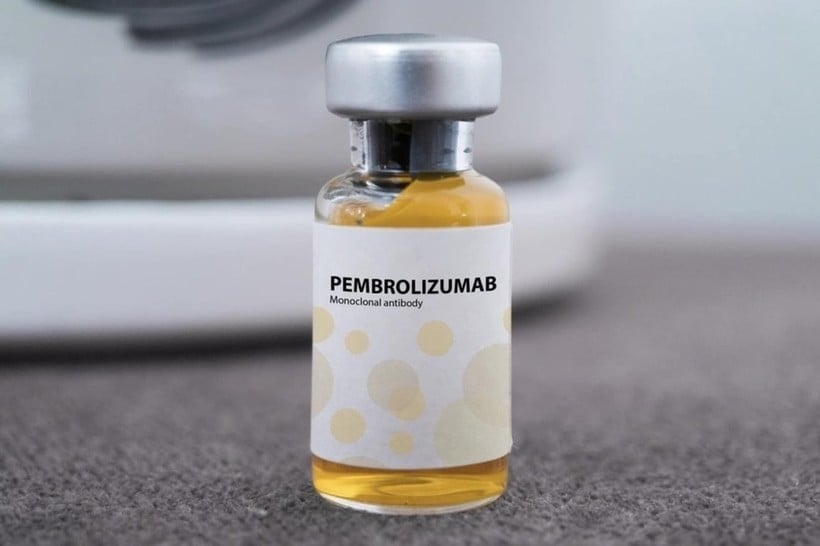
Recently, the Drug Administration of Vietnam ( Ministry of Health ) has granted a circulation registration certificate in Vietnam for an anti-cancer drug called Pembroria produced by Russia along with 13 other vaccines and biological products.
Accordingly, the official dispatch issued by the Drug Administration of Vietnam includes 14 vaccines and biological products that have been granted a 3-year registration certificate for circulation in Vietnam - batch 57. In the list attached to the above decision, there is the drug Pembroria (main active ingredient is Pembrolizumab, content 100mg/4ml) produced by Limited Liability Company "PK-137" (Russia), registered by a facility in the United Arab Emirates.
Pembroria medicine is prepared in the form of a concentrated solution for infusion, with a shelf life of 24 months from the date of manufacture.
Regarding this issue, on November 12, Professor Le Van Quang, Director of K Hospital, said that the unit will soon use this drug to treat patients. According to Professor Quang, Pembroria is not yet covered by health insurance.

Currently, Pembroria costs about 18 million VND/bottle. Patients usually use 2 bottles for 1 treatment course; lasting 12-24 courses until no longer responding to the drug, then stop. Each month, patients will be treated once.
Regarding this issue, Mr. Ta Manh Hung, Deputy Director of the Department of Drug Administration, said that in fact, many drugs with effects and indications like Pembroria have been granted circulation registration in Vietnam for a long time.
"This is not a new drug, but a product similar to the original reference biological product of MSD Pharmaceuticals (a multinational American pharmaceutical company) that has been licensed for circulation since 2017," Mr. Hung informed.
Source: https://baohaiphong.vn/thuoc-dieu-tri-ung-thu-cua-nga-su-dung-the-nao-gia-ra-sao-526432.html


![[Photo] Prime Minister Pham Minh Chinh attends a conference to review one year of deploying forces to participate in protecting security and order at the grassroots level.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/12/1762957553775_dsc-2379-jpg.webp)



![[Photo] Highways passing through Dong Nai](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/12/1762940149627_ndo_br_1-resize-5756-jpg.webp)




























































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)







Comment (0)