Recently, the news that the Ministry of Health has granted a circulation registration certificate in Vietnam for an anti-cancer drug called Pembroria, published by Dan Tri newspaper , has received special attention from the public.
Specifically, according to Decision No. 628/QD-QLD dated October 31 issued by the Drug Administration of Vietnam, Ministry of Health , Pembroria drug is granted a circulation registration certificate in Vietnam with a validity of 3 years.
Pembroria medicine has the main active ingredient Pembrolizumab, content 100mg/4ml, produced by Limited Liability Company "PK-137" (Russia), prepared in the form of a concentrated solution for infusion, with a shelf life of 24 months from the date of manufacture.

Pembroria medicine has the main active ingredient Pembrolizumab (Photo: incentra).
In addition to the above form, according to information from the circulation registration facility, Pembroria is also prepared in the form of a clear to slightly opaque, colorless to light brown solution.
What types of cancer is Pembroria indicated for?
According to the instructions for use from the registration agency, Pembroria is indicated for many types of cancer.
These are melanoma; non-small cell lung cancer; squamous cell carcinoma of the head and neck; classical Hodgkin lymphoma; urothelial cancer; esophageal cancer; colorectal cancer; non-colorectal cancer; cervical and endometrial cancer; renal cell carcinoma; triple-negative breast cancer; adenocarcinoma of the stomach or gastroesophageal junction; and cholangiocarcinoma.
Depending on the type of cancer and the stage of the disease (early or metastatic), Pembroria will be prescribed at different ages, used as monotherapy or in combination with chemotherapy and other drugs.
This medicine is used in both outpatient and hospital settings. Treatment must be initiated and supervised by experienced oncologists.
To be prescribed the above drug, patients must perform PD-L1 testing (immunohistochemistry testing to measure the amount of PD-L1 protein on the surface of cancer cells), testing to determine the tumor status of microsatellite instability (MSI) or mismatch repair (MMR) deficiency.
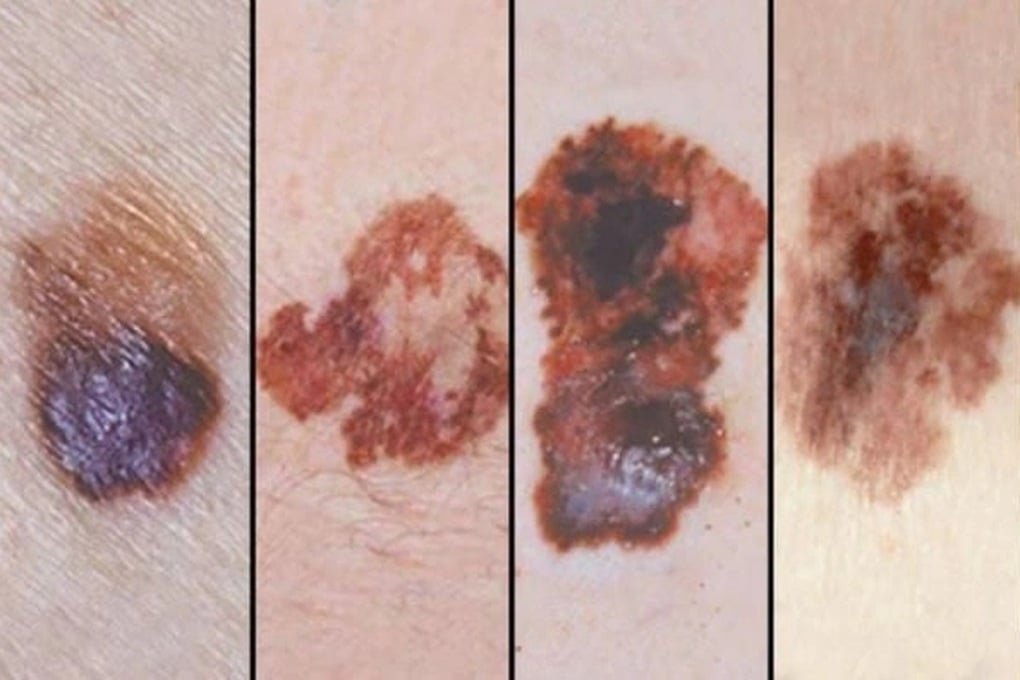
Malignant melanoma is one of the diseases that can be treated with Pembrolizumab (Photo: BV).
Notes when using
Regarding dosage, the recommended dose of Pembrolizumab in adults is 200mg every 3 weeks or 400mg every 6 weeks, given as an intravenous infusion.
The recommended dose of Pembrolizumab as monotherapy in pediatric patients 3 years of age and older (with classical Hodgkin lymphoma) or patients 12 years of age and older (with melanoma) is 2 mg/kg body weight (maximum 200 mg) administered as an intravenous infusion every 3 weeks.
Regarding combination use, it will depend on the product properties and concomitant therapies.
Depending on the cancer type, patients are advised to receive Pembrolizumab treatment until there is evidence of disease progression or unacceptable toxicity.

Cancer patients receiving treatment at Cho Ray Hospital (Photo: Hoang Le).
In the adjuvant treatment of certain cancers (such as melanoma, non-small cell lung carcinoma, or renal cell carcinoma), pembrolizumab is recommended by the manufacturer until disease recurrence, unacceptable toxicity, or for up to 1 year…
In addition to treatment indications, patients also need to pay attention to adverse immune-related reactions when using Pembroria to have appropriate adjustments, temporarily stop use or stop indefinitely.
Specifically, patients may experience conditions such as pneumonia (grade 2-3), colitis (grade 2-3), nephritis, endocrine diseases, hepatitis, severe subcutaneous reactions...
If the patient develops myocarditis, encephalitis, Guillain-Barre syndrome (acute polyneuropathy) or severe post-injection reactions (grade 3-4), treatment with this drug must be permanently discontinued.
Additionally, the safety and efficacy of pembrolizumab in people under 18 years of age have not been established (except in pediatric patients with melanoma and classical Hodgkin lymphoma). It is also recommended that the drug be administered intravenously over 30 minutes, not by rapid intravenous injection or intravenous bolus injection.
Granting of circulation registration to conduct clinical research
In Section 5, Article 2 (on the responsibilities of the manufacturing facility and the drug registration facility) of Decision No. 628/QD-QLD issued on October 31, the Drug Administration of Vietnam, Ministry of Health stated that, with drug No. 1 in the Appendix of this Decision (Pembroria), after being granted a circulation registration certificate, the company must periodically update the progress of clinical research implementation, monitor phase III immunogenicity and submit documents to change and supplement and update phase III immunogenicity monitoring data when the research period ends.
According to information from the Drug Administration, Pembroria is a monoclonal antibody, produced based on the original reference biological product of the pharmaceutical company MSD (USA). Since 2017, Keytruda - another drug with the main active ingredient Pembrolizumab - has been licensed for circulation in Vietnam.
Source: https://dantri.com.vn/suc-khoe/thuoc-pembroria-cua-nga-duoc-chi-dinh-cho-cac-loai-ung-thu-gi-20251111231728560.htm




![[Photo] Prime Minister Pham Minh Chinh attends a conference to review one year of deploying forces to participate in protecting security and order at the grassroots level.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/12/1762957553775_dsc-2379-jpg.webp)

![[Photo] Highways passing through Dong Nai](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/12/1762940149627_ndo_br_1-resize-5756-jpg.webp)































































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)







Comment (0)