Russia's Pembroria is not a new indication for cancer drugs.
On the evening of November 13, K Hospital ( Hanoi ) issued a notice on the circulation of the cancer treatment drug Pembroria (active ingredient pembrolizumab) in Vietnam.
According to K Hospital, in Vietnam, Keytruda was invented by MSD of the United States, with the active ingredient pembrolizumab, packaged in 100mg/4ml bottles and licensed for circulation by the Drug Administration of Vietnam ( Ministry of Health ) since 2017.
Pembrolizumab is essentially an anti-PD-1 monoclonal antibody and is a biological drug indicated for certain types of cancer, belonging to the group of immunotherapy. The drug is administered by intravenous infusion at a medical facility.
Keytruda is approved by the Ministry of Health for 14 indications including malignant melanoma, non-small cell lung cancer, classical Hodgkin lymphoma, urothelial carcinoma, head and neck squamous cell cancer, gastric cancer, cancer with high-level microsatellite instability, and cervical cancer.
The drug is indicated for the treatment of hepatocellular carcinoma, colorectal cancer with high-grade microsatellite instability or mismatch repair defects, primary mediastinal large B-cell lymphoma, esophageal cancer, triple-negative breast cancer, and renal cell carcinoma.
The current price of Keytruda is about 62 million VND/bottle. The drug has a partial free support program for eligible cancer patients according to Decision No. 2205/QD-BYT dated July 2, 2025 of the Ministry of Health.
The above support program is being implemented at K Hospital and many other hospitals nationwide. With this program, the maximum cost for a patient's treatment cycle is about 62 million VND with a dose of 200mg (2 bottles) and some cycles can be completely free, depending on the level of support the patient is approved for.
Meanwhile, the drug Pembroria with the active ingredient pembrolizumab, 100mg/4ml vial, manufactured by Limited Liability Company “PK-137”, Russian Federation, was granted a circulation registration certificate by the Drug Administration of Vietnam on October 31.
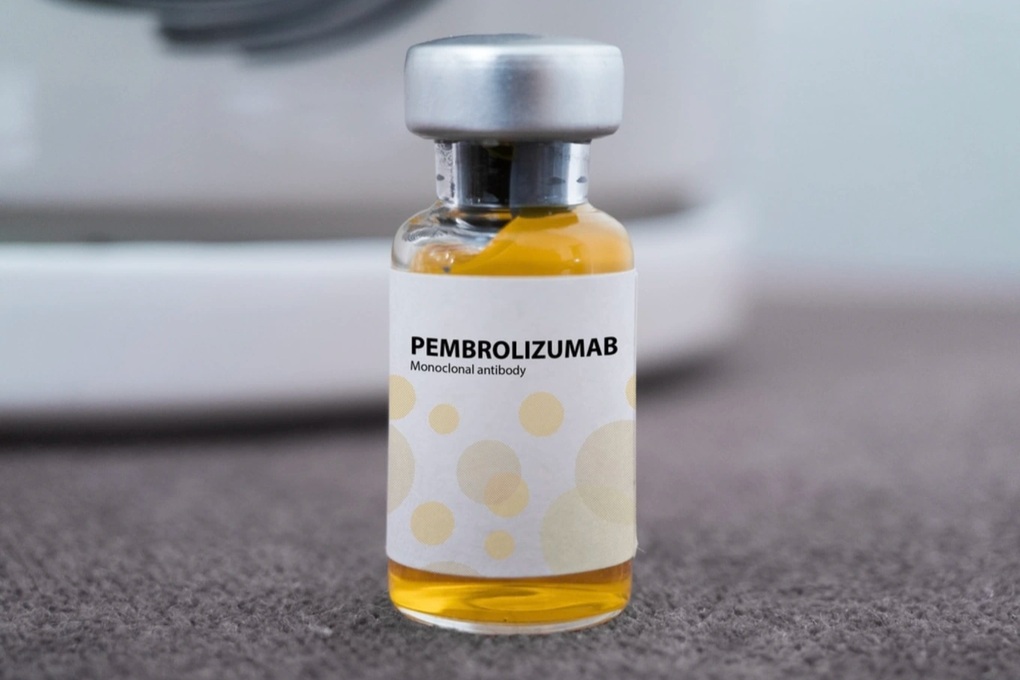
A new Russian cancer drug has been licensed for circulation in Vietnam, a course of treatment costs about 36 million VND (Photo: incentra).
Pembroria is a biologic drug with the same active ingredient as Keytruda, approved for the following indications: melanoma, non-small cell lung carcinoma, head and neck squamous cell carcinoma, classical Hodgkin lymphoma, urothelial carcinoma.
The Russian cancer drug is indicated for the treatment of esophageal carcinoma, cancer with high-grade microsatellite instability (MSI-H) or mismatch repair defects, cervical cancer, renal cell carcinoma, endometrial carcinoma, triple-negative breast cancer, adenocarcinoma of the stomach or gastroesophageal junction, cholangiocarcinoma.
The estimated price of Pembroria is about 18 million VND/bottle, with the usual dose being 2 bottles for 1 treatment cycle. Thus, the cost for a patient's treatment cycle is about 36 million VND with a dose of 200mg (2 bottles).
Currently, K Hospital is planning to purchase Pembroria medicine to use for patients in the near future.
Not all cancers require biological therapy.
K Hospital notes that not all patients with the cancers listed above are prescribed pembrolizumab.
The use of pembrolizumab also depends on many factors such as: the patient's health condition, tumor mutation type, disease stage... assessed by oncologists, individualized for each patient, to achieve the highest treatment efficiency.
K Hospital also confirmed that the Russian cancer treatment drug Pembroria is not a new cancer drug. Currently, Vietnam has nearly 100 types of monoclonal antibody drugs for cancer treatment in circulation.
Previously, the Drug Administration of Vietnam (Ministry of Health) also confirmed that the Russian cancer treatment drug that has just been licensed for circulation is not a cancer drug with a new indication.
According to Decision No. 628/QD-QLD dated October 31 of the Drug Administration of Vietnam, Pembroria was granted a circulation registration certificate in Vietnam with a validity of 3 years. The drug is prepared in the form of a concentrated solution for infusion, with a shelf life of 24 months from the date of manufacture.
It is expected that this drug will be available in the Vietnamese market in 2026. This is a biological product similar to Keytruda, with complete clinical and research records. Currently, the drug is still being monitored for immunogenicity.
Source: https://dantri.com.vn/suc-khoe/thuoc-tri-ung-thu-phien-ban-nga-se-co-tai-viet-nam-vao-nam-2026-20251113190818276.htm



![[Photo] Special class in Tra Linh](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/14/1763078485441_ndo_br_lop-hoc-7-jpg.webp)



![[Photo] Deep sea sand deposits, ancient wooden ship An Bang faces the risk of being buried again](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1763033175715_ndo_br_thuyen-1-jpg.webp)



























































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)






Comment (0)