In Europe, not all patients in every country have equal access to medicine, experts compare this situation to a " postal lottery" program.
In Romania, many drugs are distributed two years later than in Germany. If a patient in Western Europe and a patient in Eastern Europe are diagnosed with the same serious illness, they face very different outcomes, whether they live or die. Politico has compared the situation to a postal lottery.
“Patients in Western Europe and large countries have access to 90% of newly approved medicines. In Eastern Europe and smaller countries, the figure is only 10%. This is absolutely unacceptable,” EU Health Commissioner Stella Kyriakides said in a speech in April.
Senior EU officials are presenting proposals to reform the continent’s pharmaceutical market, aimed at addressing unequal access. They say companies that fail to launch products in all 27 EU markets within two years should be penalized. The goal is to create a single market that ensures all patients have timely access to safe, effective, affordable, fair and equitable medicines.
However, this is fraught with obstacles. First, there is the issue of money . In fact, some medicines are very expensive. Meanwhile, the 27 EU countries have huge economic differences, for example, Bulgaria's per capita income is almost five times lower than the Netherlands'. This means that some countries spend more on their health care systems, and in particular on medicines, than others.
Analysis of the European cancer drug market by the Swedish Institute for Health Economics (IHE) shows that the biggest spenders are Austria, Germany and Switzerland. Per capita spending on cancer drugs in these three countries is 92 to 108 euros, while the figure in the Czech Republic, Latvia and Poland is 13 to 16 euros.
Lower spending means less prescribing. For example, in the case of free cancer drugs, use in low-income countries is one-tenth to one-fifth that in rich countries.
“Due to affordability issues, a large proportion of European cancer patients, especially in Eastern Europe, cannot access effective drugs,” the researchers point out.
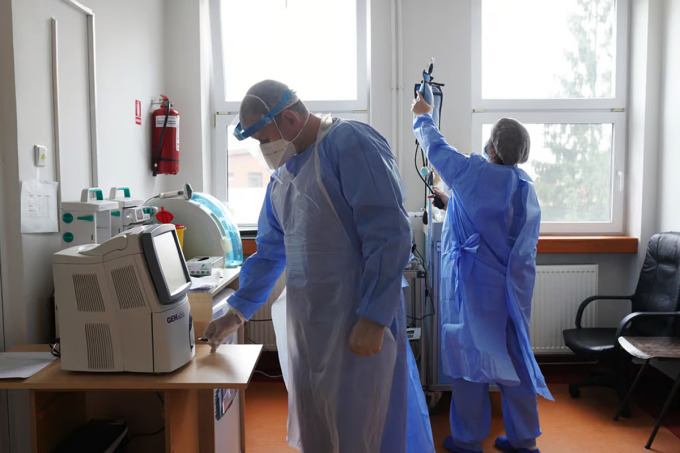
Doctors working at a hospital. Photo: Andreea Campeanu
The more complicated reason lies in the opaque and highly idiosyncratic way in which drugs are priced . To negotiate prices, pharmaceutical companies bargain directly and secretly with governments, so no country really knows what another country is paying for the same drug. This is by far the most common form.
"They (pharmaceutical companies) choose to launch new drugs in countries where they know they will pay higher prices first. In southern European countries like Portugal, Greece and Eastern Europe, drugs are proposed at a later stage, in two or three years," explains Sabine Vogler, head of pharmaceutical economics at the Austrian National Public Foundation.
Governments in high-income countries set a “reference price,” which is publicly announced. But this price is previously negotiated in secret negotiations, with an undisclosed discount. The process is repeated for subsequent countries, using the public reference price as the basis for negotiations. As a result, countries in markets deemed “less attractive” end up at the bottom.
For the pharmaceutical industry, the root cause of this process is the paperwork that forces the government to pay for a new drug.
“Applications for drug negotiations are time-consuming. Each country requires a tailored set of documents in the local language and must comply with local regulations,” the analysis, by pharmaceutical lobby group EFPIA, said.
In theory, the EU Transparency Directive requires countries to decide how much they will pay for a drug within 180 days. But in practice, in some areas, that time can be extended if regulators and governments ask companies to provide more data.
A study of the average time to access personalized cancer drugs in five countries found that Denmark takes more than four months to decide whether to pay for a drug. Poland takes up to 30 days.
The EU plans to introduce a Common Health Technology Assessment, which would give a single, bloc-wide assessment of new medicines and help countries decide how much to pay. Companies would only need to submit one set of applications, instead of 27 for each drug as they do now. The first joint assessment is expected to take place in 2025.
Countries in the Beneluxa Initiative for Pharmaceutical Policy, including Belgium, the Netherlands, Luxembourg, Austria and Ireland, have already begun to work together to buy medicines, simplifying negotiations for manufacturers and increasing the bargaining power of buyers. However, the European Commission’s proposed reform is the most ambitious.
Thuc Linh (According to Politico )
Source link


































































































Comment (0)