The Ministry of Health (Drug Administration Department) has sent a document to the Departments of Health of provinces and centrally-administered cities; and to drug manufacturing facilities regarding strengthening compliance with GMP and inspecting and monitoring production activities at drug manufacturing facilities as follows:
For pharmaceutical manufacturing facilities, raw materials must ensure strict compliance with the principles and standards of "Good Manufacturing Practice" (GMP) as prescribed by the Ministry of Health throughout the entire process of drug production, as well as the production of health supplements (if permitted to be produced on the same production line as drugs).
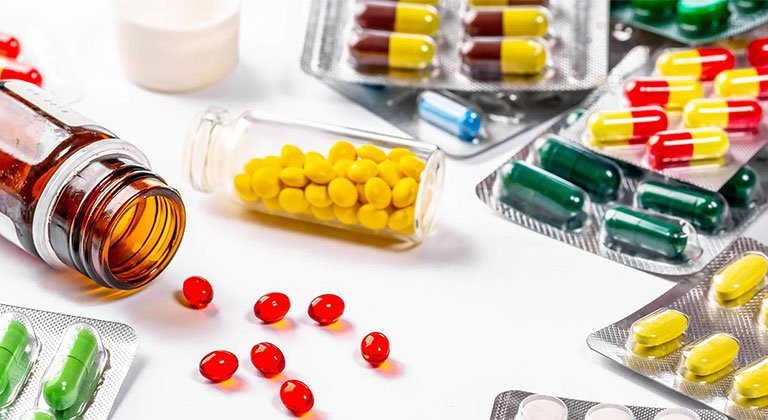
Strict control over the origin, quality, and use of pharmaceutical raw materials is essential to ensure that materials used in production are for the intended purpose, originate from the correct manufacturer as stated in the drug registration dossier, and are supplied from raw material production and distribution facilities that have undergone thorough supplier evaluation.
Raw materials for pharmaceuticals must undergo quality checks and meet the quality standards approved by the Ministry of Health in the drug registration dossier before being used in drug production.
Review the production process, production records, and testing records to ensure compliance with the production process and quality control procedures as specified in the approved and validated drug registration dossier, and to fully comply with all regulations regarding drug registration.
The Drug Administration Department clearly states that in cases of changes in the production process, quality standards, or analytical procedures to ensure the feasibility, accuracy, and precision of the method, the procedures for change must be promptly implemented according to the regulations on drug registration, and can only be implemented after being reviewed and approved by the Ministry of Health (Drug Administration Department) as prescribed.
Establish a comprehensive quality management system, controlling activities in accordance with GMP, GLP, and GSP standards; fully comply with all legal regulations in the production and business of medicines and health supplements (if any).
Strengthen self-inspection and review of compliance with GMP, GLP, GSP, and legal regulations during production and business operations, promptly detect and correct errors, and be held accountable before the law and regulatory agencies for the quality and safety of products manufactured by the facility, including pharmaceuticals and health food products.
Report promptly to the Ministry of Health and the local Department of Health when detecting issues related to the quality and safety of products manufactured by the facility.
For the Departments of Health of provinces and centrally-administered cities, the Drug Administration of Vietnam requests them to strengthen inspection and supervision of drug manufacturing facilities in their areas, especially those producing health supplements and cosmetics.
Proactively gather information and check compliance with regulations regarding production, raw material usage, labeling, advertising, etc., and maintain GMP and GSP principles in production and storage activities.
Strictly handle any violations in accordance with the law if detected, and report them to the Ministry of Health (Department of Drug Administration, Department of Traditional Medicine Management, Department of Food Safety) for timely action.
Source: https://baolaocai.vn/bo-y-te-kiem-soat-chat-nguon-goc-chat-luong-va-viec-su-dung-nguyen-lieu-lam-thuoc-post402764.html




![[Photo] Prime Minister Pham Minh Chinh presides over a meeting on private sector economic development.](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F20%2F1766237501876_thiet-ke-chua-co-ten-40-png.webp&w=3840&q=75)





























































































Comment (0)