The Ministry of Health is drafting a Circular regulating the list of tests, paraclinical services and conditions for using paraclinical results when connecting results between medical examination and treatment facilities.
The draft Circular proposes regulations on the list of tests, paraclinical services and conditions for using paraclinical results when connecting results between medical examination and treatment facilities, applicable at medical examination and treatment facilities nationwide.
Principles of using test results
According to the draft, medical examination and treatment facilities shall self-assess publicly the quality of testing and the professional management agency shall announce that they have achieved quality level 1 or higher according to the provisions of Decision No. 2429/QD-BYT dated June 12, 2017 of the Ministry of Health on promulgating criteria for assessing the quality level of medical laboratories, interconnection, and recognition of results according to the principle: mutual recognition of results between laboratories achieving the same quality level; laboratories achieving a low quality level shall recognize the results of laboratories achieving a higher quality level.
For laboratories that have achieved ISO 15189, apply the interoperability of testing techniques in the accredited list corresponding to ISO 15189.

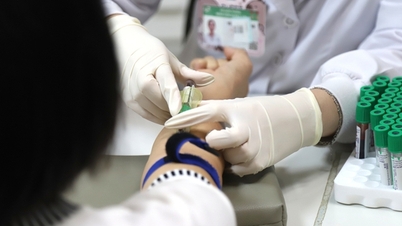
Taking samples for testing for patients at Bach Mai Hospital.
The doctor examining and treating the patient is the one who decides whether to use the test results or whether it is necessary to order a re-test depending on the patient's medical condition and clinical progression.
Principles of using electro-optical results
The draft clearly states the use value of radiographic results when transferring medical examination and treatment facilities.
Diagnostic imaging results (digital data, descriptive results, conclusions) are part of the medical record and have legal value when: Performed at a licensed medical examination and treatment facility operating within the scope of expertise; have the signature and name of the performing physician and the diagnosing physician (paper copy) or digital signature/electronic authentication (electronic copy).
When transferring to a medical examination and treatment facility, the receiving facility has the right to use the results for reference and as a basis for treatment, and is not required to redo the test/imaging diagnosis if the quality and legality are guaranteed.
Requirements for medical image data
The image must be intact, clear, complete in sequence/pulse (for CT, MRI), and complete in time (for DSA).
DICOM standard format, with reading software or integrated in PACS.
Image data connecting PACS–PACS between hospitals requires patient information, identification code, and scan time.
According to the draft, the facility transferring the radiological results is responsible for sending the result form, electronic image data along with the medical record when transferring the patient. Ensure that the patient's information on the image data matches the medical record. Do not arbitrarily edit or crop the image data.
Rights of the facility receiving radiographic results
The facility receiving the X-ray results may reuse the imaging results for diagnosis and treatment if they meet the requirements. They are allowed to use them for further treatment, but have the right to decide to retake the images when necessary.
Have the right to request re-imaging in the following cases: Clinical and paraclinical changes that are not consistent with the previous imaging and radiation stage; the time from imaging to reception is too long, no longer consistent with the patient's condition; poor image quality, not complete in the necessary sequence; there is suspicion of incorrect patient information.
The recommended list of biochemical testing techniques has 40;
The recommended list of hematology laboratory techniques is 55;
The recommended list of microbiological testing techniques is 26;
The proposed list of Genetic-Molecular Biology testing techniques is 86;
The proposed list of Pathological Testing Techniques is 14;
The proposed Electro-Optical Engineering category is 893.
The Ministry of Health is soliciting comments on this draft on the Ministry of Health's Electronic Information Portal.
Source: https://suckhoedoisong.vn/bo-y-te-de-xuat-lien-thong-gan-1100-danh-muc-cac-xet-nghiem-dich-vu-can-lam-sang-giua-cac-benh-vien-169251120083630218.htm


![[Photo] President Luong Cuong receives President of the Senate of the Czech Republic Milos Vystrcil](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F20%2F1763629737266_ndo_br_1-jpg.webp&w=3840&q=75)

![[Photo] Lam Dong: Panoramic view of Lien Khuong waterfall rolling like never before](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F20%2F1763633331783_lk7-jpg.webp&w=3840&q=75)
![[Photo] National Assembly Chairman Tran Thanh Man holds talks with South Korean National Assembly Chairman Woo Won Shik](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F20%2F1763629724919_hq-5175-jpg.webp&w=3840&q=75)



































































































Comment (0)