VISTA-1 study on oral immunotherapy drug RBS2418, developed by the US biopharmaceutical company Riboscience, with the participation of experts from Stanford University (USA). VISTA-1 was approved by the US Food and Drug Administration (FDA) in September 2024 and licensed by the Vietnamese Ministry of Health in December 2024.

Dr. Vu Huu Khiem advises patients on the conditions and procedures for participating in the VISTA-1 study.
Photo: Tam Anh General Hospital
In the phase 1 study in the US, RBS2418 recorded initial data on safety and tolerability. In phase 2A, the study continued to evaluate the drug's safety and potential efficacy. This is the first time Vietnam has participated in a clinical study of a cancer drug right from phase 2A as the main research point.
Choosing Tam Anh as Stanford's overseas center for cancer research and clinical trials was not a random choice. In particular, Tam Anh General Hospital has qualified experts and modern lab equipment to perform "central testing" on-site without having to send test samples abroad as before.
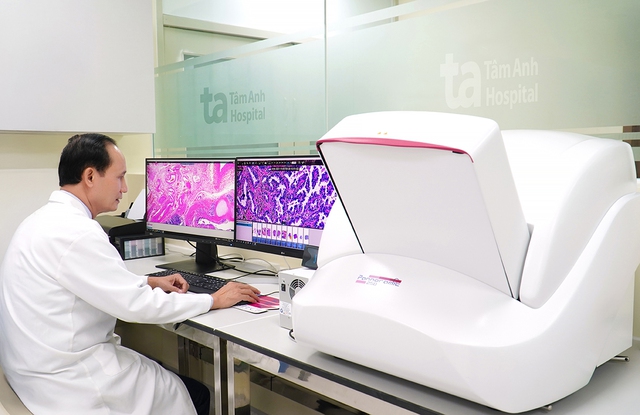
The Histech 3D Slide Scanner uses artificial intelligence to scan and digitize entire histological slides with extremely high resolution, an important tool for evaluating biomarkers in the VISTA-1 study.
Photo: Tam Anh General Hospital
Up to now, after 3 months, Tam Anh General Hospital has received and screened hundreds of patients, of which 8 are being treated according to the VISTA-1 research protocol.
Dr. Vu Huu Khiem, Head of the Oncology Department of Tam Anh General Hospital, Hanoi , and the main researcher, said that the patients are being closely monitored for safety and response to the research drug. The research team has not recorded any significant abnormal symptoms related to the research drug in patients who have used the drug in the past 3 months.
"For patients with advanced colorectal cancer who no longer respond to current treatment regimens, each improvement in survival time during the research process is very meaningful. We and the patients have now entered the fourth month of implementing this research," said Dr. Vu Huu Khiem.

Patients participating in the VISTA-1 study received comprehensive care during treatment.
Photo: Tam Anh General Hospital
Mr. C., 75 years old, was diagnosed with stage 3 colorectal cancer in 2023, had chemotherapy, surgery, and then treated lung metastasis with radiofrequency ablation and chemicals. The disease continued to progress, he was prescribed targeted therapy but had to stop after a week due to side effects. As soon as the VISTA-1 study started in Vietnam, he registered at Tam Anh General Hospital in Hanoi and became one of the few elderly patients eligible to participate.
" The research drug is taken orally so it can be used at home, without needing to be hospitalized for infusion like with conventional immunotherapy drugs. This is very convenient, especially for the elderly, those who have difficulty traveling and live far from the hospital like me ," said Mr. C. He still has normal daily activities and has not recorded any significant abnormalities during the follow-up after 1 month of treatment in the research.
VISTA-1 is the first international study in the field of cancer that Tam Anh General Hospital System participated in right from phase 2A, which is the stage of evaluating the effectiveness of the invented drug. To prepare for this important study, Tam Anh General Hospital System and Tam Anh Research Institute (TAMRI) have invested heavily to develop a central laboratory that meets ISO 15189 standards for the fields of Hematology - Biochemistry - Microbiology and Molecular Biology of the Ministry of Health .
The Labo is equipped with many modern specialized devices for research, supporting the assessment of the expression levels of two biological markers ENPP1 and cGAS in tissue samples. This is the first time these technologies have been deployed in the clinical research system at Tam Anh General Hospital. Tam Anh General Hospital is also the unit that has been transferred the testing techniques for two new biological markers ENPP1 and cGAS, thereby qualifying to participate in the VISTA-1 study.

Ventana Bench Mark Ultra immunohistochemistry and chromogenic fluorescence in situ hybridization (ISH) analyzer (USA) helps detect and differentiate many types of cancer.
Photo: Tam Anh General Hospital
According to Dr. Phuong Le Tri, Executive Director of Tam Anh Research Institute (TAMRI), in the past, Vietnamese patients, especially those with serious illnesses such as cancer, had little opportunity to access information and participate in new drug research, and often had to go abroad to seek opportunities.
Vietnam's participation in international research such as VISTA-1 helps patients access advanced drugs right in the country, while opening up opportunities for Vietnamese doctors and scientists to participate in professional research processes, and gain early access to professional research processes and world-leading innovative drugs.
The VISTA-1 study continues to recruit patients.
The VISTA-1 study is currently recruiting patients in Vietnam and the US until the required number is reached. Participants will be regularly checked for health and disease progression; supported with research-related costs, travel support, and 24/7 consultation from a team of experts.
The Oncology Department of Tam Anh General Hospital in Hanoi and Tam Anh General Hospital in Ho Chi Minh City opened a clinic called "VISTA-1 Research Consultation", offering free consultation and checking eligibility for research participation. If you or your loved ones would like to learn more about the clinical research on RBS2418, please contact: cskh@tahospital.vn
Source: https://thanhnien.vn/nhung-benh-nhan-viet-nam-thu-nghiem-thuoc-chua-ung-thu-cua-my-co-tin-hieu-tot-185250505192008858.htm



![[Photo] Prime Minister Pham Minh Chinh meets with South African President Matamela Cyril Ramaphosa](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/23/1761226081024_dsc-9845-jpg.webp)

![[Photo] President Luong Cuong holds talks with South African President Matamela Cyril Ramaphosa](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/23/1761221878741_ndo_br_1-8416-jpg.webp)























































































Comment (0)