On August 22, the Drug Administration of Vietnam ( Ministry of Health ) announced a list of nearly 800 types of drugs and pharmaceutical ingredients produced domestically and abroad that have been newly granted or have their circulation registration certificates extended in Vietnam to serve the needs of disease treatment and prevention.
Accordingly, 432 domestically produced drugs were granted new circulation registration certificates; 69 domestically produced drugs were granted circulation registration certificates; 292 foreign drugs were granted circulation registration certificates and 4 drugs with proven bioequivalence were granted circulation registration certificates.
Of the 432 domestic pharmaceutical products newly granted circulation registration certificates, 422 drugs were granted circulation registration certificates for 5 years; the remaining 10 products were granted circulation registration certificates for 3 years.
Of the 69 domestically produced drugs whose circulation registration certificates have been extended, 44 drugs and pharmaceutical ingredients have been granted circulation registration certificates for 5 years; 20 drugs and pharmaceutical ingredients have been granted circulation registration certificates for 3 years; and the remaining 5 products have circulation registration certificates valid until December 31, 2025.
Of the 229 foreign drugs granted registration extensions on this occasion, 187 drugs were granted 5-year extensions; 38 drugs were granted 3-year extensions and 1 drug product was granted an extension until December 31, 2025.
The Drug Administration of Vietnam requires that drug manufacturing and registration establishments are responsible for manufacturing drugs in accordance with the records and documents registered with the Ministry of Health and must print or affix the registration number issued by the Vietnamese Ministry of Health on the drug label; Only manufacture and put into circulation special control drugs when having a Certificate of eligibility for pharmaceutical business with a scope of business of special control drugs suitable to the scope of operation.
The Drug Administration of Vietnam requires drug registration establishments to ensure the maintenance of pharmaceutical business conditions as prescribed in Point b, Clause 2, Article 42 of the Law on Pharmacy during the validity period of the drug circulation registration certificate, drug ingredients...
The domestically and internationally produced pharmaceutical products that have been newly granted or have their circulation registrations renewed this time are quite diverse in terms of pharmacological effects, including: drugs for treating respiratory tract infections, drugs for treating osteoarthritis, drugs for treating cardiovascular diseases, hypertension, diabetes, cancer treatment, antiviral drugs, antibiotics, pain relievers, anti-inflammatory drugs, bioequivalent drugs.../.
Source: https://www.vietnamplus.vn/bo-y-te-cap-moi-gia-han-giay-dang-ky-luu-hanh-gan-800-loai-thuoc-post1057159.vnp



![[Photo] Prime Minister Pham Minh Chinh chairs the conference to review the 2024-2025 school year and deploy tasks for the 2025-2026 school year.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/22/2ca5ed79ce6a46a1ac7706a42cefafae)
![[Photo] President Luong Cuong receives delegation of the Youth Committee of the Liberal Democratic Party of Japan](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/22/2632d7f5cf4f4a8e90ce5f5e1989194a)

![[Photo] President Luong Cuong attends special political-artistic television show "Golden Opportunity"](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/22/44ca13c28fa7476796f9aa3618ff74c4)


















































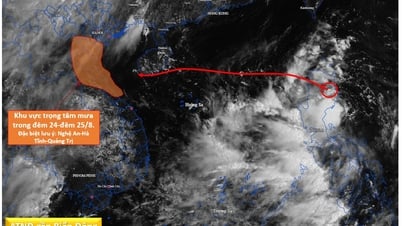














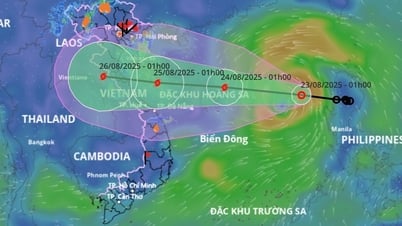




















Comment (0)