The Ministry of Health has just issued Document No. 2349 BYT-QLD on warning about some cough syrup products that are banned from use to the Department of Health of provinces and centrally run cities; Medical examination and treatment facilities under the Ministry of Health .
Accordingly, the Ministry of Health said it had received a telegram from the International Criminal Police Organization (Interpol) warning Interpol member agencies about hundreds of children who had died or suffered acute kidney damage after using 14 syrup products banned in several countries.
| STT | Product Name | Manufacturer | Affected countries | Area |
|---|---|---|---|---|
| 1 | Promethazine Oral Solutinon | MAIDEN PHARMACEUTICALS LIMITED | Gambia | Africa |
| 2 | Kofexmalin Baby Cough Syrup | MAIDEN PHARMACEUTICALS LIMITED | Gambia | Africa |
| 3 | Makoff Baby Cough Syrup | MAIDEN PHARMACEUTICALS LIMITED | Gambia | Africa |
| 4 | Magrip N Cold Syrup | MAIDEN PHARMACEUTICALS LIMITED | Gambia | Africa |
| 5 | Termorex Syrup | PT KONIMEX | Indonesia | Southeast Asia |
| 6 | Flurin DMP Syrup | PT YARINDO | Indonesia | Southeast Asia |
| 7 | Unibebi Cough Syrup | PT UNIVERSAL PHARMACEUTICAL INDUSTRIES | Indonesia | Southeast Asia |
| 8 | Unibebi Demam Paracetamol Drops | PT UNIVERSAL PHARMACEUTICAL INDUSTRIES | Indonesia | Southeast Asia |
| 9 | Unibebi Demam Paracetamol Syrup | PT UNIVERSAL PHARMACEUTICAL INDUSTRIES | Indonesia | Southeast Asia |
| 10 | Paracetamol Drops | PT AFI FARMA | Indonesia | Southeast Asia |
| 11 | Paracetamol Syrup (mint) | PT AFI FARMA | Indonesia | Southeast Asia |
| 12 | Vipcol Syrup | PT AFI FARMA | Indonesia | Southeast Asia |
| 13 | Ambronol Syrup | MARION BIOTECH PVT.LTD | Uzbekistan | Central Asia |
| 14 | DOK-1 MAX Syrup | MARION BIOTECH PVT.LTD | Uzbekistan | Central Asia |
According to information from Interpol, these products manufactured in India and Indonesia contain Diethylene which can lead to serious health damage or death to users.
To ensure safety for users, the Ministry of Health requests the Department of Health of provinces and centrally-run cities, medical examination and treatment facilities under the Ministry of Health to notify medical facilities, pharmaceutical facilities in the area, departments and rooms at the unit about the warning information for the 14 cough syrup products mentioned above to warn about the serious harm if using the product and strictly prohibit its use.
The Department of Health of provinces and centrally-run cities shall conduct inspections and checks at pharmaceutical business establishments on the circulation of these products in particular and drugs of unknown origin and origin, and drugs that have not been licensed for circulation in general on the market.
In case these products are found to be in circulation, recall, destroy and handle violations by pharmaceutical businesses according to regulations to avoid causing harm to users.
Previously, in October 2022, the World Health Organization (WHO) announced that dozens of young children in Gambia, a country in West Africa, died from acute kidney injury possibly related to contaminated cough and cold syrup produced by an Indian drug manufacturer.
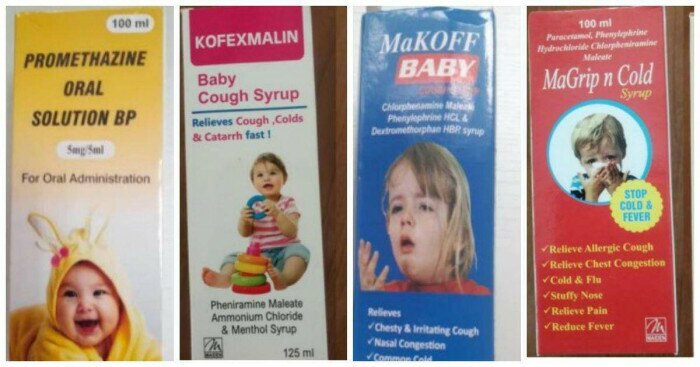
4 types of syrups WHO has just issued a warning about the dangers that may be related to deaths in children
This warning covers four products: Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup.
Laboratory analysis found “unacceptable” levels of diethylene glycol and ethylene glycol. These substances are highly toxic and can cause death or side effects such as abdominal pain, vomiting, diarrhea, headaches… which can be toxic and lead to acute kidney injury.
The Ministry of Health said it had received the information and had reviewed the list of drugs imported into Vietnam. The review results showed that Vietnam had not yet issued any registration numbers to Maiden Pharmaceuticals Ltd.; had not yet issued registration numbers to four cough medicine products: Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup. At the same time, Maiden Pharmaceuticals Ltd. also did not have any documents submitted to the health authority.
Source


































































































Comment (0)