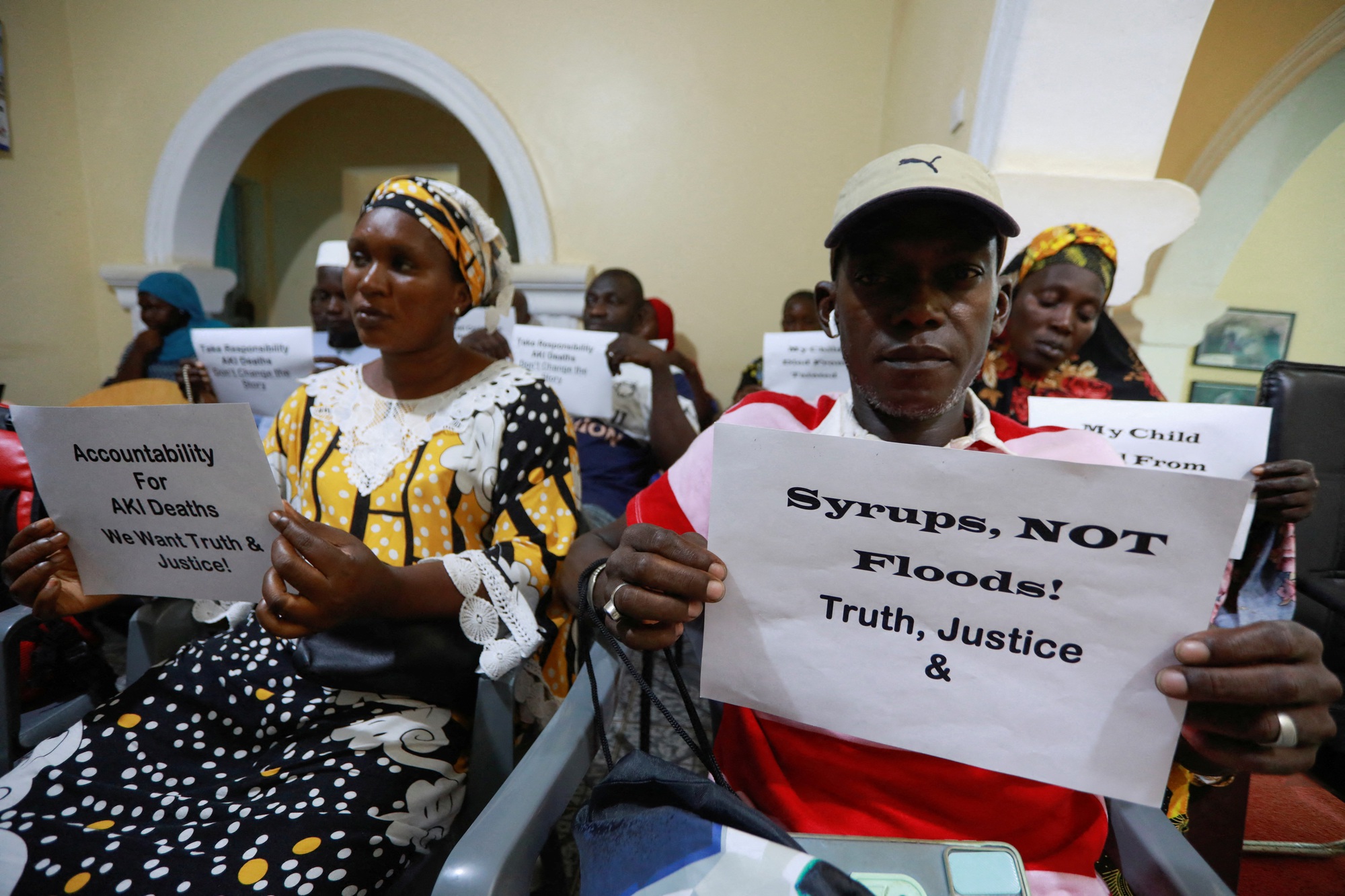
Parents of children who died from cough syrup-related illnesses at a press conference in Gambia
Reuters reported on July 25 that India has just suspended the manufacturing license of a pharmaceutical company after the World Health Organization (WHO) spoke out about a cough syrup product containing a dangerous ingredient discovered in the Marshall Islands and Micronesia in April.
Indian regulators are investigating drugmakers after a cough syrup made in the country was linked to at least 89 child deaths in Gambia and Uzbekistan last year.
The move is an effort to protect India's image as the "pharmacy of the world " providing affordable medicines globally.
Child dies from cough syrup, parents, doctors fight to demand government action
WHO has warned that samples taken from a batch of cough syrup from QP Pharmachem Company (headquartered in Punjab, India) contained unacceptable levels of diethylene glycol and ethylene glycol, which can cause poisoning and even death.
QP Pharmachem Ltd has denied all allegations and said it plans to appeal the suspension.
Meanwhile, Deputy Health Minister Bharati Pravin Pawar, when reporting to parliament, said that drug samples taken from manufacturing facilities were concluded to be "not of standard quality".
She added that the authorities have suspended the manufacturing and export licenses of QP Pharmachem Ltd and two other companies whose products have been linked to the child deaths: Maiden Pharmaceuticals and Marion Biotech Pvt. Ltd.
QP Pharmachem CEO Sudhir Pathak confirmed that the company has stopped production. He said he tested the ingredients before starting production of the syrup.
He also said that he only exports products to Cambodia and does not know how the products can reach the Marshall Islands and Micronesia.
India has tightened checks on cough syrup exports since June, requiring companies to obtain a certificate of analysis from a government laboratory before exporting.
Source link




































































































Comment (0)