On May 29, the Drug Administration of Vietnam ( Ministry of Health ) issued a document requesting the Hanoi Department of Health to urgently coordinate with the police, market management, Steering Committee 389 and relevant agencies to inspect and examine An An pharmacy (address: Residential group 14, Kien Hung ward, Ha Dong district, Hanoi).
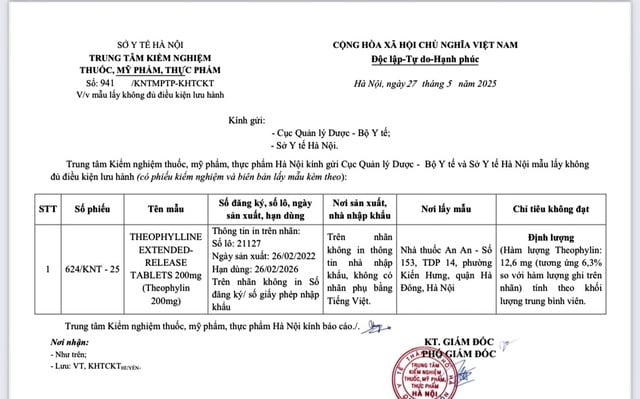
Theophylline sample contains only 6.3% of the content stated on the label.
PHOTO: DEPARTMENT OF DRUG ADMINISTRATION
Previously, the Drug Administration received a report from the Hanoi Center for Drug, Cosmetic and Food Testing that the product sample had information printed on the label: Theophylline Extenderdrelease tablets 200mg (theophyllin 200mg), batch number 21127, manufacturing date 26.2.2022, expiration date 26.2.2026; place of manufacture Pharmacy Laboratories Plus, without information about the circulation registration number, import license number, and importing facility on the label.
The drug sample did not meet the quality requirements for theophylline quantification, only reaching 6.3% of the content stated on the label.
The drug sample was taken by the Hanoi Center for Drug, Cosmetic and Food Testing at An An Pharmacy at the above address.
The Drug Administration of Vietnam requested the Hanoi Department of Health to strictly handle violating establishments according to regulations and at the same time trace the origin of the batch of drug products that do not meet quality requirements.
According to information from the treatment unit, Theophylline Extended is a bronchodilator drug used to treat asthma and chronic obstructive pulmonary disease.
Source: https://thanhnien.vn/truy-tim-thuoc-theophylline-chi-co-63-ham-luong-hoat-chat-185250529114534949.htm
































































































Comment (0)