 |
| A series of eye infections caused by contaminated eye drops have been reported in the US. (Source: AdobeStock/mala) |
Serious consequences
CDC statistics show that as of May 18, 81 people in 18 US states have been infected, 5 people have died (including 4 people who had to have their eyeballs removed) and 14 people have lost their vision.
Pseudomonas aeruginos is an aerobic Gram-negative bacillus that causes many different types of infections in humans such as intestinal infections, urinary tract infections, pneumonia, dermatitis, attacks wounds and surgical incisions of patients and causes severe sepsis.
Pseudomonas aeruginos bacteria are common in natural environments, such as soil and water, and are commonly spread in healthcare settings. In 2017, it caused more than 32,000 infections in hospitalized patients and about 2,700 deaths in the United States.
Signs of an eye infection may include: Yellow, green, or clear discharge from the eyes; possible pain or discomfort in the eye, redness of the eye or eyelid, foreign body sensation, sensitivity to light, and blurred vision.
CDC is currently working with the U.S. Food and Drug Administration (FDA) and state and local health departments to investigate this outbreak of extensively drug-resistant Pseudomonas aeruginosa. The new outbreak strain, called VIM-GES-CRPA, has not been reported in the United States before.
The CDC is particularly concerned and warns that Pseudomonas aeruginos can spread from people using contaminated eye drops to people who do not use the drops.
Series of products not meeting quality standards
In the United States, these infections have been linked to 10 brands of eye drops, the most common being EzriCare artificial tears from Global Pharma Healthcare. The eye drops are distributed by EzriCare and Delsam Pharma.
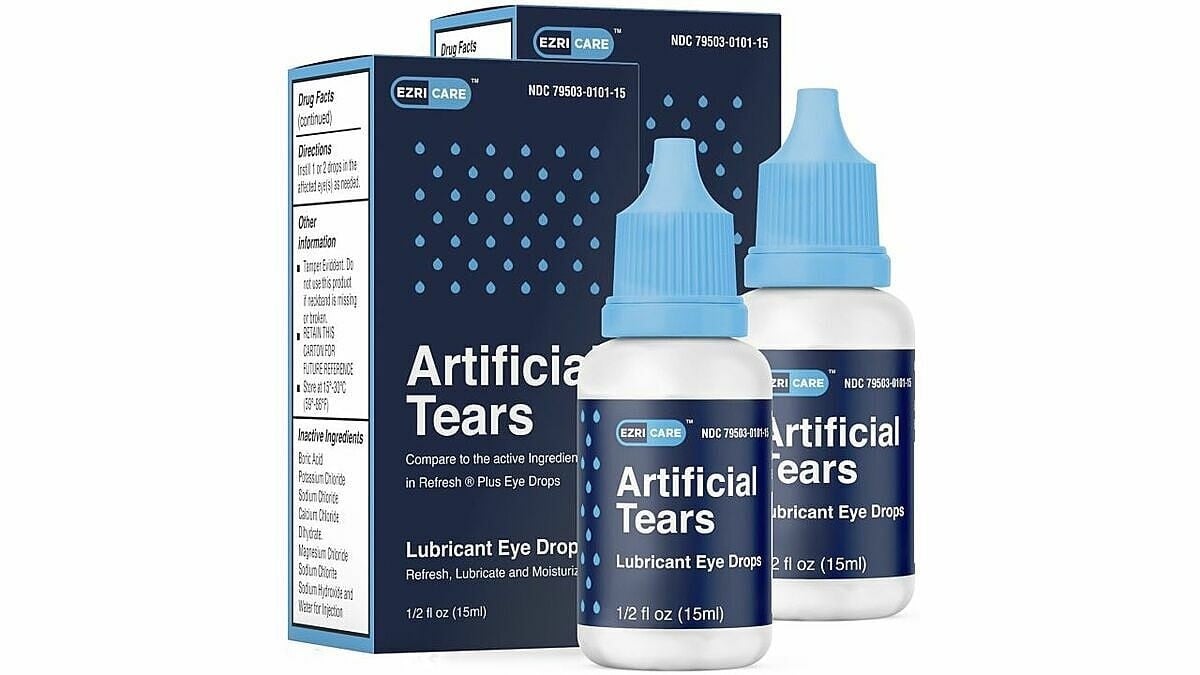 |
| EzriCare artificial tears brand. (Source: EzriCare) |
The outbreak has been linked to a variety of infections, including eye infections. The investigation identified EzriCare artificial tears as a common exposure for many patients.
EzriCare artificial tears, a nonprescription, preservative-free product packaged in multi-dose bottles, was the most commonly reported contaminated product.
CDC laboratory testing identified the presence of VIM-GES-CRPA bacteria in opened bottles of EzriCare from different lots collected from patients with and without eye infections in two U.S. states.
The FDA tested unopened bottles of EzriCare artificial tears and also identified contamination with this bacteria.
The FDA encourages health care professionals and patients to report complications or quality problems with any drug to the agency's MedWatch program. Consumers can also report adverse drug reactions by contacting the FDA Consumer Complaint Coordinator.
Previously, in February, Global Pharma Healthcare's EzriCare artificial tears product was recalled due to poor quality assurance.
According to the FDA report, the agency inspected the production process of the Global Pharma factory for 11 days. The results showed that the production process of the product batch from December 2020 to April 2022 did not ensure the level of sterility.
CDC warning
Upon receiving information about the infections, CDC and FDA recommended that clinicians discontinue prescribing and patients discontinue use and discard EzriCare artificial tears and two additional products manufactured by the same manufacturer, Delsam Pharma artificial tears and Delsam Pharma ophthalmic ointment, until further guidance is available.
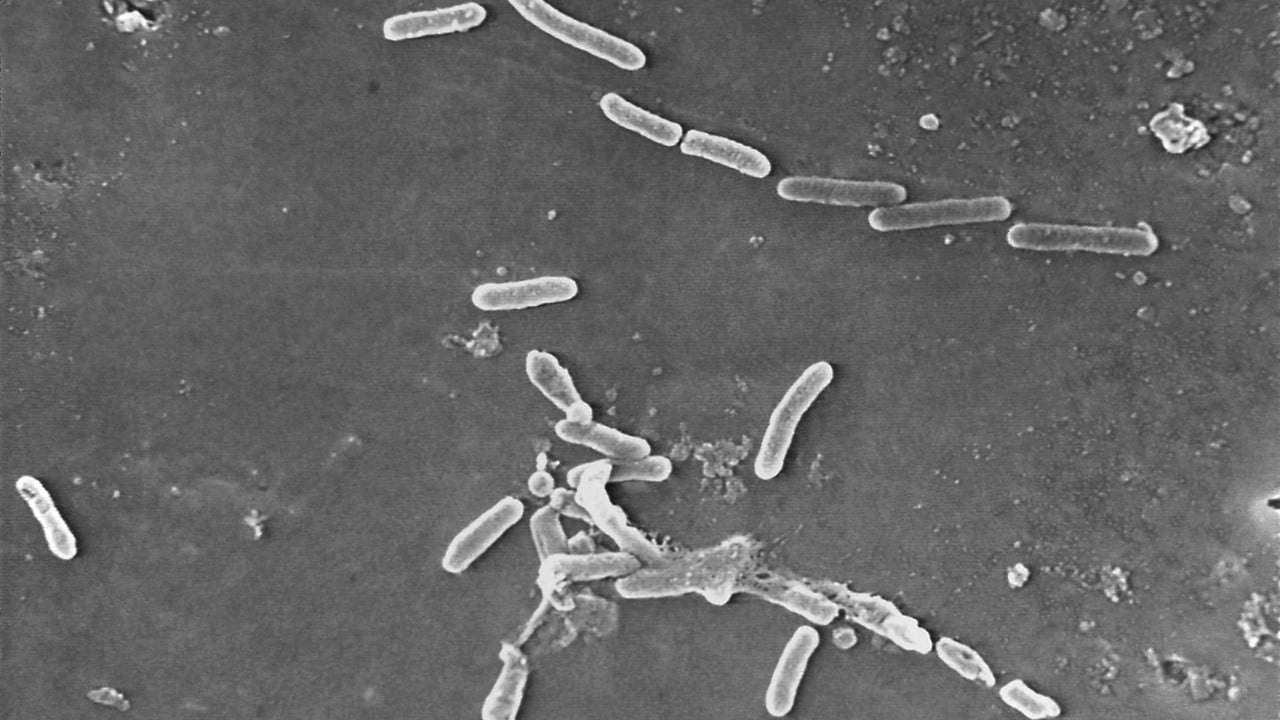 |
| Electron microscopic image of rod-shaped Pseudomonas aeruginosa bacteria. (Source: US CDC) |
According to the CDC, the VIM-GES-CRPA strains associated with this outbreak were extensively resistant to drugs including cefepime, ceftazidime, piperacillin-tazobactam, aztreonam, carbapenems, ceftazidime-avibactam, ceftolozane-tazobactam, fluoroquinolones, polymyxin, amikacin, gentamicin, and tobramycin.
However, a small group of three VIM-GES-CRPA isolates appeared to be susceptible to cefiderocol. Nevertheless, clinicians should consult with experts to determine the treatment plan for patients infected with this widely resistant organism.
Because of concerns about the spread of this bacteria in healthcare settings, CDC recommends that health care providers caring for patients with Pseudomonas aeruginosa VIM-GES-CRPA infection follow infection control recommendations to prevent cross-infection to other patients, as these bacteria have the potential to spread rapidly in healthcare settings.
CDC recommends isolating any patient infected with VIM-GES-CRPA in a health care setting and using contact precautions.
Source


































































































Comment (0)