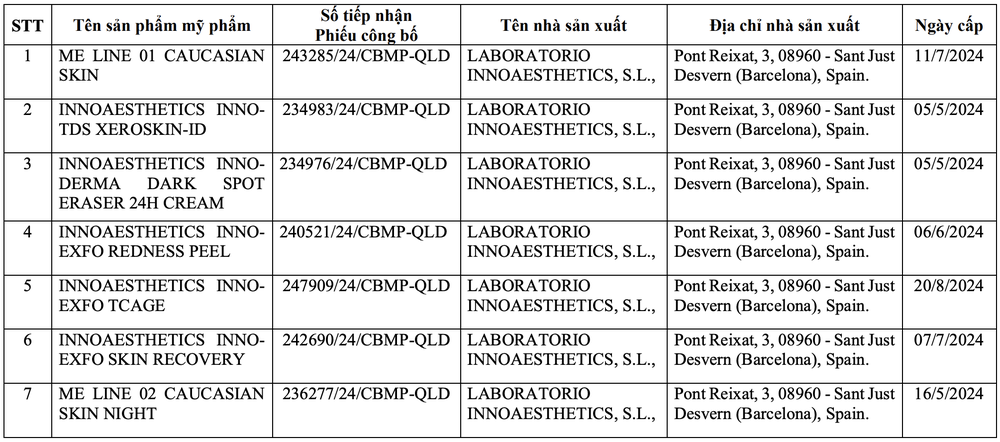
7 imported cosmetic products were suspended from circulation and recalled nationwide due to formulas not being as declared in the dossier.
The Department of Pharmacy ( Ministry of Health ) has just sent a document to health departments regarding the suspension of circulation and nationwide recall of 7 products that Dong Nam Global Pharmaceutical Company Limited is responsible for bringing to the market.
The business address listed above is at No. 12, Lane 40 Nguyen Chinh, Tan Mai Ward, Hoang Mai District, Hanoi ; business registration number: 0110539718.
These 7 products have formulas that are not as stated in the published documents.
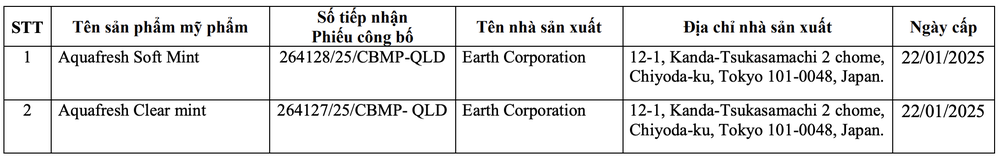
Along with that, the Drug Administration has issued a document on suspending circulation and recalling nationwide two products produced by Phat Anh Minh Company Limited.
Address declared on the announcement file at No. 13D, Information Command collective area, Tu Hiep commune, Thanh Tri district, Hanoi; business registration number: 0106208110.
The reason for the recall is that the cosmetics in circulation have labels that do not meet product labeling regulations.
The Drug Administration of Vietnam requests health departments to notify cosmetic businesses and users in the area to immediately stop selling and using the above products and return them to the suppliers; inspect and handle violators according to current regulations.
Dong Nam Global Pharmaceutical Company Limited and Phat Anh Minh Company Limited must send recall notices to distributors and users of the above-mentioned imported cosmetic products; receive returned products from business establishments and proceed to recall and destroy all products that do not meet regulations.
The Drug Administration of Vietnam has decided to temporarily stop reviewing and receiving cosmetic product declaration dossiers for Dong Nam Global Pharmaceutical Company Limited and Phat Anh Minh Company Limited for 6 months, starting from July 2.
The application for a cosmetic product declaration receipt number submitted by the above two companies before July 2 is no longer valid.
Source: https://baolaocai.vn/dinh-chi-thu-hoi-tren-toan-quoc-loat-my-pham-nhap-khau-post647837.html






















![[Photo] Prime Minister Pham Minh Chinh attends the opening ceremony of the National Data Center](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/18/b5724a9c982b429790fdbd2438a0db44)












































































Comment (0)