Under the new recommendations, Leqembi will only be prescribed to early-stage Alzheimer's patients who carry the ApoE4 gene - an important risk factor for Alzheimer's disease.
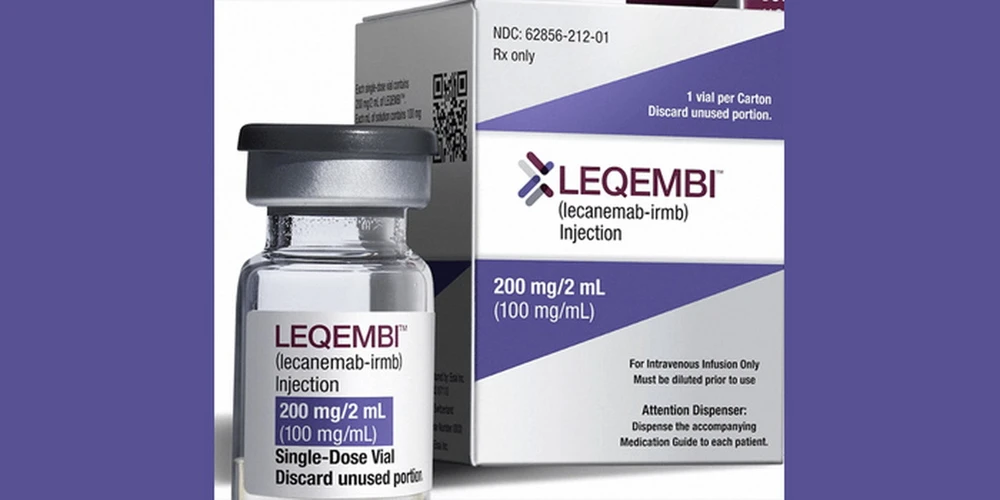
On November 14, the European Medicines Agency (EMA) officially recommended the licensing of Leqembi for the treatment of early-stage Alzheimer's disease. This decision marks an important milestone in the fight against this degenerative neurological disease.
The EMA had previously rejected Leqembi in July due to concerns about serious side effects such as brain swelling. However, after a more thorough assessment, the EMA's Committee for Medicinal Products for Human Use (CHMP) reversed its decision.
Under the new recommendations, Leqembi will only be prescribed to early-stage Alzheimer's patients who carry the ApoE4 gene - an important risk factor for Alzheimer's disease.
The study showed that in this group of patients, the benefits of Leqembi in slowing disease progression outweighed the risks.
The final decision on whether to grant a marketing authorisation for Leqembi across the European Union will be taken by the European Commission. Once approved, each member state will decide on its own pricing and health insurance coverage for the medicine.
Leqembi is a product developed and manufactured by two leading pharmaceutical companies, Eisai (Japan) and Biogen (USA). The drug works by removing beta-amyloid plaques, a toxic protein that accumulates in the brains of Alzheimer's patients./.
Source: https://www.vietnamplus.vn/eu-bat-den-xanh-cho-viec-su-dung-thuoc-leqembi-dieu-tri-alzheimer-post993619.vnp

































































































Comment (0)