Two hospitals, the 108 Military Central Hospital and Bac Kan General Hospital, have informed that two types of milk produced by these companies have won bids to supply the hospitals and have been advising patients to use them over the past period.
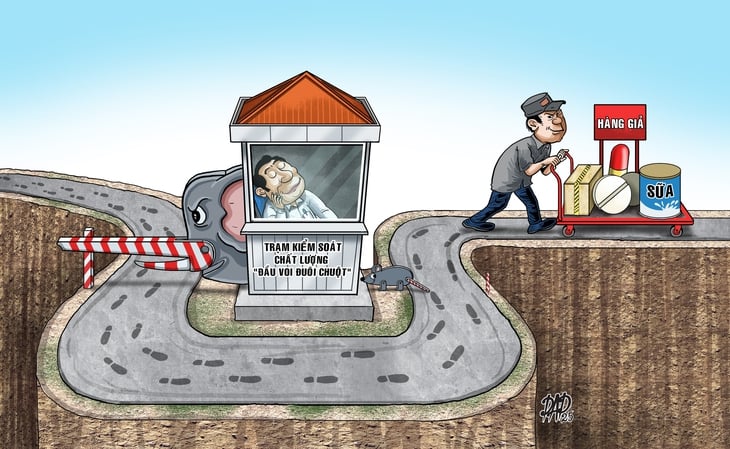
Fake milk enters hospitals despite "correct" bidding
Both hospitals confirmed that "this product was put into use after a bidding process in accordance with legal regulations".
According to Dr. Nguyen Huy Hoang (Vietnam - Russia High Pressure Oxygen Center, Ministry of National Defense ), the story of milk from a fake milk production company appearing from the market to hospitals has shown loopholes.
The products were advertised with "rare ingredients" such as bird's nest and cordyceps, but when tested, they were found to be completely non-existent. The actual nutritional content was less than 70% - enough to be considered counterfeit under Vietnamese law.
"What is especially dangerous is that these products are brought into hospitals through legal bidding channels, proving that the production - marketing - document legalization network has operated extremely systematically and bypassed many supervision stages.
Through this "incident", it can be seen that compliance with the bidding process does not mean quality assurance. A poor quality product can still win the bid if the technical documents are just a formality. Contractors are mainly approved on paper, without actual capacity testing. There is no quality inspection of the batch after winning the bid.
In reality, the import process is mainly based on quantity, expiry date, and labels, but there is no independent testing system, especially for products "outside the health insurance list" that patients pay for themselves, Dr. Hoang said.

Consumers must be protected by law from the jungle of dairy products. In the photo: a milk shop in front of the hospital gate - Illustration: CHI TUE
Does the hospital have to test after receiving goods?
According to Dr. Nguyen Huy Hoang, after this incident, it is necessary to establish a post-import testing process before using it on patients. At the same time, increase the role of the drug and treatment council in approving products. Build an internal warning system, receive doubts from doctors and patients, and review and re-evaluate all suppliers.
In addition, for state management agencies, clearly define the responsibilities between ministries in the management of medical nutrition and functional foods. Strengthen post-inspection, periodic inspection and public disclosure of results; apply technology to trace the origin of products in hospitals and pharmacies.
Mr. Dao Xuan Co, director of Bach Mai Hospital, said that the hospital does not have the function of testing goods, requiring the authorities to check the quality of goods, from import, production to circulation to ensure. So that when the goods arrive at the hospital, doctors and patients can use them with peace of mind.
In addition, the head of the unit must also be "honest" so as not to be bribed by businesses, so that low-quality, highly discounted counterfeit goods cannot enter the hospital.
"In reality, patients have to bear an additional burden related to functional foods. The psychology of patients is that whatever the doctor prescribes, they often have to try to buy it," said Mr. Co.
According to Mr. Co, at Bach Mai Hospital, doctors are not allowed to prescribe or advise on functional foods, and the hospital pharmacy does not sell functional foods.
"This has been done at the hospital for 3 years now. In treating diseases, treatment methods and drugs are key factors. Doctors can advise on additional daily nutrition for patients.
Prescribing additional functional foods will increase the financial burden for patients, while the effectiveness is unclear," said Mr. Co.

Inside the "ecosystem" of fake milk production and trading - Photo: CAND Newspaper
"Loophole" of Decree 15
According to the Food Safety Departments (FSDs), nutritional products are made according to the provisions of Decree 15 by submitting declaration dossiers and self-declaring products. Facilities only have to submit test certificates of safety indicators issued by the Ministry of Health according to the principle of risk management.
The Decree does not require quality index testing certificates. This also leads to the situation where businesses do not test product quality indexes when submitting documents, because it is not mandatory.
Speaking to Tuoi Tre , Mr. Vu Duc Toan, head of the inspection and professional department of the Hoa Binh Province Food Safety Department, said that the post-inspection of products is based on "risk factors". Meanwhile, these products are registered locally but there are no products in the locality, and the department has not received feedback from the people so it does not post-inspecte the products.
In Hanoi, although in 2023 the inspection team randomly took samples of products in the warehouse: 4 samples from Rance Pharma and 1 sample from Hacofood.
However, the post-inspection work is based on the guidance of Decree 15, checking which factors directly affect people's health, specifically safety indicators. Therefore, in this inspection, the test results of all samples met safety indicators.
It can be seen that the current post-inspection and food quality management only focus on controlling safety indicators (microbiological and heavy metal indicators) from pre-inspection to post-inspection and preventing hazards (testing to prevent the use of prohibited substances in food) at the post-inspection stage.
In the assessment of the impact of Decree 15, the Food Safety Department also acknowledged that although post-inspection work is still maintained, the work of inspection, examination, and post-inspection of food safety only partially meets actual requirements.
Besides, according to current regulations, post-audit also has its own regulations, it cannot be done "at any time" but must have a plan.
Post-inspection is usually carried out according to the annual plan; surprise inspections will be carried out when there are signs of violations, complaints, or feedback from consumers, or at the request of specialized inspection work, or according to the surprise decision of the competent authority.
After 7 years of implementation, Decree 15 has revealed many loopholes in the management of self-declared products and submission of declaration dossiers.
Decree 15 is being revised.
Currently, the Food Safety Department is seeking opinions on amending Decree 15, including some amendments to strengthen post-inspection with the aim of controlling and further improving food quality and controlling organizations and individuals who register the declaration or self-declare the product along with its features and uses; and changing the product after the product declaration.
In particular, for the ingredients of products belonging to the health protection food group, the formula will be required to only include the ingredients that create the product's effects and the ingredients that help stabilize the product's formula (antioxidants, avoiding interactions and incompatibilities with the ingredients that create the effects)...
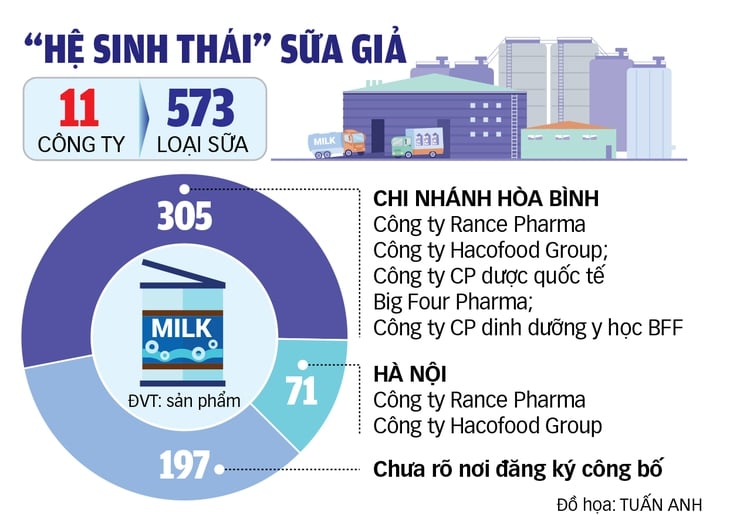
There is no list of products manufactured by the 11 companies yet.
April 11: Ministry of Public Security dismantled 11 companies - producing 573 brands of various types of powdered milk
April 14: Ministry of Industry and Trade said "not subject to management"
April 15: Ministry of Health reviews products published elsewhere
April 17: Prime Minister Pham Minh Chinh signed and issued dispatch No. 40 on handling the production and distribution of fake milk.
April 20: Ministry of Health reviews prescription and milk advertising consultation at medical facilities
To date, there is no list of products manufactured by the 11 companies.
Europe and America: Milk management: strict and rigorous

Dairy products counter at a supermarket in Europe - Photo: DAIRY HERD
* In Europe: Known as one of the most stringent and demanding markets in the world, Europe has enacted a food safety and quality control system for all dairy products.
The European Union's General Food Law (GFL) places primary responsibility on food and feed producers to ensure food safety at all stages – including production, processing and distribution from farm to fork.
Member states are responsible for enforcing the regulation, as well as monitoring and ensuring businesses comply with the law, according to information from the European Dairy Association (EDA).
Thomas Linsinger, scientific officer for health and consumer affairs at the European Commission (EC), said in January that the EC's Joint Research Centre had developed a new reference material called ERM-BD519 to test the purity of milk fat and detect products adulterated with cheap fat.
This achievement provides laboratories with a reliable tool to perform ISO 17678/IDF 202 - the EU-recognized milk quality testing standard.
In addition, the EU established the Rapid Alert System for Food and Feed (RASFF) in 1979.
This is an online data and alert tool that allows consumers free access to look up information on food and feed safety between EU countries, thereby preventing unsafe products from reaching the market or reaching consumers.
In addition, EU product packaging regulations require dairy products to have clear and accurate information, helping consumers make smarter purchasing decisions and preventing fraud.
* In the US: The US Food and Drug Administration (FDA) is responsible for setting all standards for the composition, quality, and labeling of milk and dairy products.
In addition, the FDA is also responsible for enforcing these regulations through regular inspections of milk processing facilities, testing milk samples, and monitoring the labeling and advertising of these products.
In addressing the issue of adulterated milk, the FDA will first gather information on trends and cases of milk fraud in the marketplace from multiple sources. It will then conduct testing on suspected milk products and test new food fraud detection tools, including chemical and biological methods.
In addition, the agency also regularly samples many dairy products on the market to check their quality, according to the FDA's official website.
In addition to the FDA, the US Department of Agriculture (USDA) has a role in issuing legal frameworks, promoting and researching milk, monitoring product quality through a classification and standardization system, to manage the US milk market more closely, according to the FDA's official website.
Source: https://tuoitre.vn/hang-gia-long-hanh-bit-ngay-lo-hong-20250421081852799.htm


![[Photo] Prime Minister Pham Minh Chinh receives the Chairman of the Japan-Vietnam Friendship Association in the Kansai region](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/03/1762176259003_ndo_br_dsc-9224-jpg.webp)
![[Photo] Fall Fair 2025 and impressive records](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/03/1762180761230_ndo_br_tk-hcmt-15-jpg.webp)
![[Photo] General Secretary To Lam receives Singaporean Ambassador Jaya Ratnam](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/03/1762171461424_a1-bnd-5309-9100-jpg.webp)



































































































Comment (0)