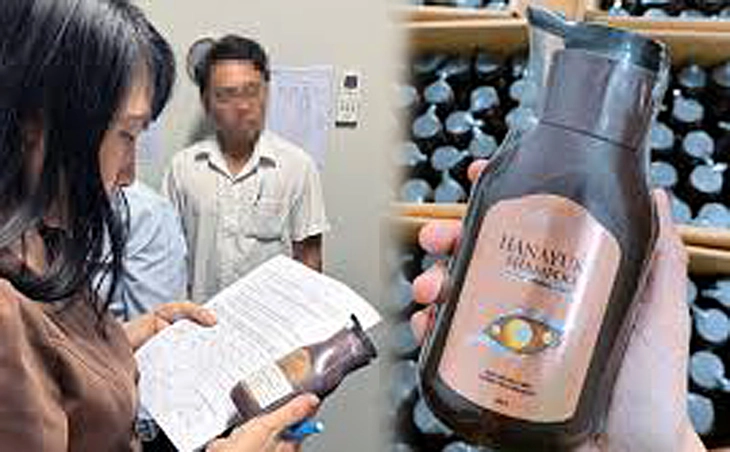
Hanayuki Shampoo by Doan Di Bang has been recalled due to violating safety standards - Photo: AB
What is Phenoxyethanol? Does it affect health that makes many brands "hide" it?
A series of products do not meet microbiological standards.
Recently, many cosmetic products have been recalled, causing confusion among consumers. Among them, products distributed by Doan Di Bang have emerged.
Hanayuki Shampoo product - 300g bottle, manufactured by EBC Dong Nai Medical Factory Joint Stock Company and widely promoted by singer Doan Di Bang, was suspended from circulation by the Drug Administration ( Ministry of Health ), requested to be recalled and destroyed nationwide.
The reason is that the product does not meet microbiological quality standards and also contains 2-phenoxyethanol - a preservative not included in the officially announced formula, violating the law on cosmetic declaration.
Next, another product of Doan Di Bang, the G-Thera Amino Anti-Wrinkle Mask, was also recalled. The reason given was that the original label of the product did not list the correct ingredients as in the cosmetic product declaration file (missing butylene glycol, phenoxyethanol, ethylhexylglycerin).
Also related to this preservative, the product Adaphil Gentle Skin Cleanser - 125ml bottle with the brand name GAMMA - was also recalled. Test results showed that the product sample contained two preservatives, methylparaben and propylparaben, which were not listed in the product declaration file approved by the Drug Administration.
In October 2024, the Drug Administration of Vietnam also decided to suspend the circulation, recall and destroy two Cerina products under the GAMMA brand because they contained the ingredient 2-phenoxyethanol, which was not declared in the cosmetic declaration formula.
Recently, the Drug Administration has continuously recalled cosmetics that violate the law. Many consumers are concerned about what this substance is and whether it affects their health, which has caused many brands to "hide" the presence of this substance in their products.
How can it affect?
According to Dr. Vo Thi Doan Phuong - Head of Clinical Department 3, Ho Chi Minh City Dermatology Hospital, phenoxyethanol or 2-phenoxyethanol is an aromatic glycol ether used with many different functions and applications, such as a preservative in pharmaceuticals, cosmetics and personal care products; a bactericide in disinfectants (human hygiene products) or a solvent in formulations (coatings, functional liquids)...
They have a broad spectrum of antibacterial activity effective against a wide range of Gram-negative and Gram-positive bacteria as well as against yeasts and have only a weak inhibitory effect on the skin microflora.
According to the European Scientific Committee on Consumer Safety, phenoxyethanol is safe for all consumers (including children of all ages) when used as a preservative in cosmetic products at a maximum concentration of 1%.
Adverse systemic effects (on blood, liver) have been observed in animal toxicity studies but only at exposure levels many times higher (approximately 200 times higher) than those to which consumers are exposed when using cosmetic products containing phenoxyethanol.
Although widely used in cosmetic products, phenoxyethanol is a rare sensitizer. France recommends against the use of phenoxyethanol as a preservative in cosmetic products intended for use on the diaper area (including wipes) of infants and children under 3 years of age.
"We enjoy good health thanks to a balanced and healthy microbiome in our bodies and on our skin. The microbiome is an essential partner to our skin.
It plays a major role in protecting our skin and regulating the exchange between the body and the environment.
Its balance is essential for the health and beauty of the skin. Thus, if we use cosmetics with many microorganisms and harmful microorganisms, our skin will become weakened, the body's protective function will be damaged and we will get diseases caused by these harmful microorganisms," Dr. Phuong explained and warned.
Using poor quality cosmetics has many potential risks.
According to the European Chemicals Agency (ECHA), 2-phenoxyethanol may cause skin and eye irritation or allergic reactions in some people, especially those with a history of allergies or sensitive skin. This ingredient declaration helps consumers to be aware and avoid using it if they are sensitive to this substance.
In addition, for special groups such as pregnant women, breastfeeding women or infants, disclosure of ingredients helps them make appropriate decisions about use because this substance can affect the central nervous system of infants if used incorrectly.
Sharing about the widespread use of fake and poor quality cosmetics, Mr. Le Huu Doanh - Director of the Central Dermatology Hospital - also said that during the examination and treatment process, doctors have encountered many patients with complications due to using cosmetics and skin care products of unknown origin.
Common cases are contact dermatitis, irritation, or reactions to pharmaceutical and chemical ingredients that the user is unaware of because they are not clearly stated on the product label.
"Skin care cosmetics not only have a beauty role but also contribute to protecting the skin and health. Therefore, choosing products with clear origin and quality control is extremely important in beautifying and treating skin diseases," Mr. Doanh shared.
Dr. Vu Thai Ha - Head of the Department of Research and Application of Stem Cell Technology, Central Dermatology Hospital - also said that cheating and not clearly stating the ingredients in cosmetics is very dangerous. "When the ingredients are not clearly known, users do not know the dosage. Overdosing on some active ingredients can cause systemic diseases, not just the outer skin area."
What is the safety margin?
According to Circular 06/2011 on cosmetics management in Vietnam, manufacturers and distributors must fully disclose the list of product ingredients when registering for circulation.
In particular, 2-phenoxyethanol is a chemical preservative that needs to be clearly listed so that the authorities can check whether the concentration used complies with the safety limit (usually below 1%). Similarly, international standards such as the FDA (USA) or EC 1223/2009 (Europe) also require the ingredients to be disclosed to ensure consumer safety.
The declaration of 2-phenoxyethanol also helps regulators and manufacturers monitor the quality of the product throughout its life cycle. This ensures that the preservative concentration is always within safe limits, especially when the product is mass produced or imported.
Source: https://tuoitre.vn/phenoxyethanol-trong-dau-goi-nha-doan-di-bang-la-gi-ma-giau-nhem-2025060322373624.htm































































































Comment (0)