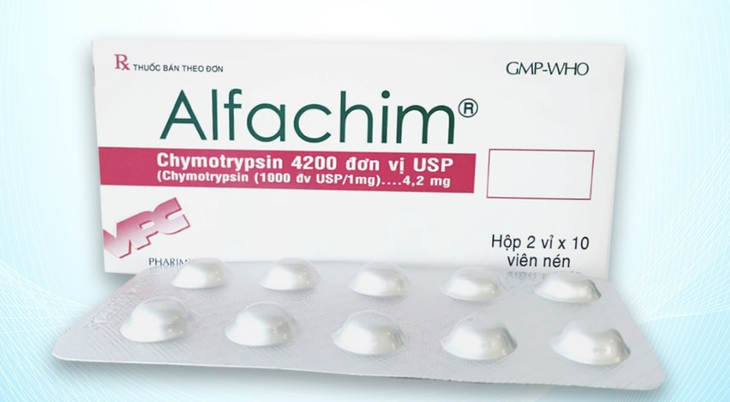
Alfachim 4.2 tablets (Chymotrypsin equivalent to 21 microkatal chymotrypsin, 4200 IU) are manufactured by Cuu Long Pharmaceutical Joint Stock Company.
The Drug Administration of Vietnam ( Ministry of Health ) has just decided to recall nationwide the batch of Alfachim 4.2 tablets (Chymotrypsin equivalent to 21 microkatal chymotrypsin, 4200 IU), registration number VD-34573-20, batch number 03010624, manufactured by Cuu Long Pharmaceutical Joint Stock Company.
Previously, on May 15, the Department of Drug Administration decided to recall the above Alfachim 4.2 tablets in Hanoi .
The reason for the recall is based on the test report of the Central Institute for Drug Control stating that the above batch of drugs did not meet quality requirements.
Afterwards, the Drug Administration requested Cuu Long Pharmaceutical Company to coordinate in taking two additional samples to send to the testing agency to re-check the quality according to quantitative criteria.
However, although it has been more than 15 days since the Drug Administration Department issued a dispatch requesting coordination, Cuu Long Pharmaceutical Company has not reported additional testing results, but has only sent information related to the production, distribution and recall of the product. The reason is that the testing units currently have many samples to send, so the waiting time for testing is longer.
Therefore, this enterprise then submitted a document requesting a voluntary recall of the batch of Alfachim 4.2 that violated the law. Based on this request and the above reasons, the Drug Administration Department issued a decision to recall the batch of medicine nationwide.
According to the decision of the Department, Cuu Long Pharmaceutical Joint Stock Company must urgently coordinate with the drug distribution system, send recall notices to all wholesale and retail establishments using Alfachim 4.2 products of lot 03010624.
The entire recall process must be implemented within 2 days from the date of signing the document, reporting the recall results, including the quantity produced, distributed, the amount of drugs recalled, along with evidence of implementation at each facility, within 18 days.
The Drug Administration of Vietnam requests the Department of Health of provinces and cities, as well as health sectors, to widely announce the recall to users and update the recall information on the official website.
Relevant units need to closely monitor the recall process, strictly handle cases of intentional non-compliance, and report the results to the Department of Drug Administration for timely handling.
As for the Hanoi Department of Health and the Vinh Long Department of Health - where the headquarters and manufacturing plant of Cuu Long Pharmaceutical Company are located - the authorities requested to strengthen supervision of drug recalls and assess the thoroughness of this activity.
If the violating product is still able to continue to circulate and be used on the market and can adversely affect the health of users, the units need to report immediately for handling measures.
The discovery and recall of a batch of substandard drugs shows that the drug quality monitoring system is working.
Alfachim 4.2 is a drug containing the enzyme Chymotrypsin, often indicated in the treatment of inflammation and edema due to trauma or after surgery. Using drugs that do not ensure the active ingredient content can affect the treatment effectiveness.
Source: https://tuoitre.vn/thu-hoi-toan-quoc-doi-voi-lo-thuoc-vien-nen-alfachim-4-2-20250622164728559.htm



![[Photo] Phu Quoc: Propagating IUU prevention and control to the people](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/24/f32e51cca8bf4ebc9899accf59353d90)




![[Photo] Party and State leaders meet with representatives of all walks of life](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/24/66adc175d6ec402d90093f0a6764225b)






























































































Comment (0)