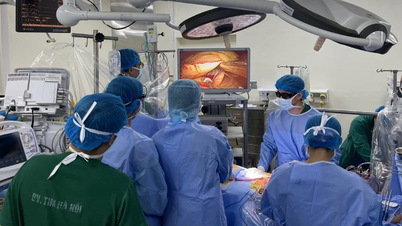
The data shows a large disparity in access to new medicines across countries, reflecting differences in institutions, approval capacity, and R&D investment. Vietnam is among the countries with low access, posing an urgent need for policy reform to help people quickly access the latest medical advances.
 .JPG) |
To help people have the opportunity to improve their health through access to new, advanced medicines, some countries have special support mechanisms.
According to the IQVIA MIDAS Report, in Hong Kong, regulatory improvements have accelerated the approval process for innovative medicines and raised the assessment standards to a level closer to that of internationally recognized regulatory authorities such as the United States, Canada, Europe, Japan and Australia. The expansion of the “1+” approval mechanism, which allows for the approval of evidence-based medicines registered in a single reference country, together with expanded health insurance coverage, are contributing to improved access to innovative pharmaceutical products.
Similarly, the Japanese Ministry of Health , Labor and Welfare (MHLW) has implemented an “early launch” incentive and tax exemption for intellectual property, starting from April 2025 for a period of seven years. Adjustments related to the price maintenance mechanism and post-launch incentives will help protect the prices of innovative drugs throughout the patent validity period. In addition, the fast-track approval mechanism under the Sakigake system has also been formalized to enhance accessibility.
The problem of drug access in Vietnam is not simply a market issue, but lies in the clinical trial mechanism, approval policies and internal capacity of the domestic pharmaceutical industry. As the population aging rate increases – forecast to reach 25% by 2040, and the rate of non-communicable diseases accounts for 77% of the disease burden – delays in drug access not only reduce the quality of life, but also put a burden on the health system and the state budget.
Therefore, the Healthcare Innovation Forum held tomorrow, June 6, is an important opportunity for policy makers, management agencies, domestic and foreign experts, pharmaceutical enterprises, technology enterprises, medical examination and treatment facilities, etc. to come together to find the most effective solutions for healthcare for Vietnamese people, helping people receive better healthcare.
 |
At the forum, guests will analyze and propose solutions from policy to technology. In particular, AI is expected to be a tool to help optimize the data screening process, predict drug efficacy, and increase feasibility in clinical trial design - helping to reduce costs and drug development time by 30 - 50% compared to present.
Vietnam needs a complete R&D ecosystem, from laboratory infrastructure, financial policies to encourage business investment, to a transparent legal corridor and international standards. Tomorrow's forum will not only "talk about solutions" - but will be a place where solutions are co-created so that Vietnamese people can soon access advanced global medicine.
Source: https://baodautu.vn/doi-moi-the-che---chia-khoa-de-nguoi-dan-co-co-hoi-su-dung-thuoc-tien-tien-d297761.html



![[Photo] National Assembly Chairman Tran Thanh Man holds talks with New Zealand Parliament Chairman](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/c90fcbe09a1d4a028b7623ae366b741d)
![[Photo] General Secretary To Lam attends the opening ceremony of the National Achievements Exhibition](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/d371751d37634474bb3d91c6f701be7f)

![[Photo] Politburo works with the Standing Committee of Cao Bang Provincial Party Committee and Hue City Party Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/fee8a847b1ff45188749eb0299c512b2)
![[Photo] Red flag with yellow star flutters in France on National Day September 2](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/f6fc12215220488bb859230b86b9cc12)
![[Photo] General Secretary To Lam presents the 45-year Party membership badge to comrade Phan Dinh Trac](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/e2f08c400e504e38ac694bc6142ac331)




















































































Comment (0)