New Hope for Lung Cancer Patients: New Drugs Are Safe, Effective, and Affordable for Clinical Trials |
The findings were published in the New England Journal of Medicine (NEJM) on April 28 and presented at the American Association for Cancer Research annual meeting in Chicago the same day.
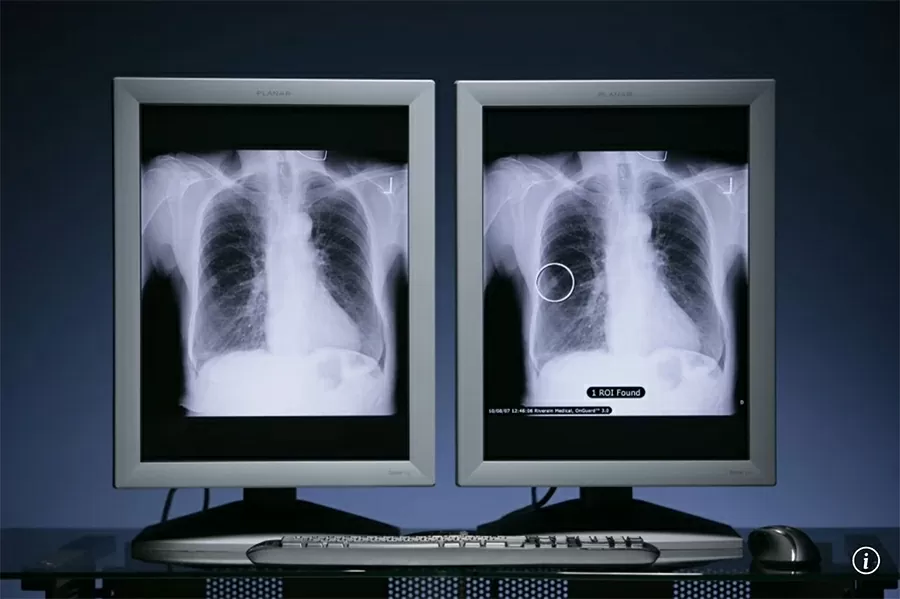
Researchers found that the oral drug zongertinib was more effective than standard treatment in treating HER2-mutant non-small cell lung cancer (NSCLC). The drug works by targeting the HER2 protein and blocking the activity of the protein’s tyrosine kinase, which is responsible for transmitting cell growth signals.
This type of lung cancer is particularly difficult to treat. Unlike other variants of NSCLC, there is only one treatment option approved by the U.S. Food and Drug Administration to date: intravenous antibody-drug conjugate (ADC) therapy. And ADCs carry the risk of side effects, including diarrhea, nausea, fatigue, and skin rashes.
According to Professor Wang Xin of the Cancer Hospital of the Chinese Academy of Medical Sciences, zongertinib has the "potential to set a new benchmark for targeted therapy in the treatment of HER2-mutant NSCLC."
The multi-center study was conducted at 82 sites worldwide , including 18 sites in the United States, 17 sites in China, and three sites in Japan. The analysis published by NEJM included 188 NSCLC patients, with a predominance of Asian populations.
In the trial, treatment with zongertinib, jointly developed by Boehringer Ingelheim and Sino Biopharmaceutical Limited, resulted in better clinical outcomes across multiple endpoints compared with standard ADC therapy.
Lung cancer is the most common cancer worldwide. Last year, there were an estimated 2.5 million new cases globally, with 1.8 million related deaths. |
Among patients treated with zongertinib, 71% had tumor shrinkage to target size, compared with only 49% in patients treated with ADCs.
Another important measure of how well a cancer drug works is progression-free survival, which is the amount of time the treatment stops the cancer from growing. Zongertinib stopped lung cancer from growing for an average of 12.4 months, while drugs in the same class of ADCs stopped tumor growth for an average of 9.9 months.
Finally, lung cancer patients treated with zongertinib experienced significantly fewer serious side effects than those undergoing ADC therapy. Among patients receiving ADCs, 17% to 26% experienced severe diarrhea, compared with only 1% of those receiving zongertinib.
Due to the lack of a control group in the phase 1 drug trial, the ongoing phase 3 clinical trial will further confirm the efficacy and safety of zongertinib in the first-line treatment of patients with HER2-mutant non-small cell lung cancer (NSCLC).
A total of 169 hospitals participated in the phase 3 clinical trial, including 18 hospitals in the United States, 5 hospitals in Japan, and 25 hospitals in China.
China has surpassed the United States to become the global center for clinical trials of new drugs, according to the SCMP.
Li Jin, director of the oncology department at the East Hospital affiliated to Tongji University in Shanghai, noted that the average cost per subject to complete a clinical trial can be as high as $500,000, while in China it is only about $41,000 while still ensuring quality.
Source: https://baoquocte.vn/hy-vong-moi-cho-benh-nhan-ung-thu-phoi-thuoc-moi-an-toan-hieu-qua-voi-muc-chi-phi-thu-nghiem-lam-sang-hop-ly-313983.html























![[Photo] National Assembly Chairman Tran Thanh Man visits Vietnamese Heroic Mother Ta Thi Tran](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/7/20/765c0bd057dd44ad83ab89fe0255b783)









































































Comment (0)