“A quantum leap” over conventional treatment
In the treatment of multiple myeloma (bone marrow cancer), CAR-T therapy - with T cells carrying modified antigen receptors - is highly effective when the disease relapses or does not respond to other treatments.
However, the traditional CAR-T treatment process is very complicated: T cells need to be taken from the patient, then modified and cultured outside the body, and finally infused back into the patient. This process is often time-consuming, expensive and requires high technical requirements.
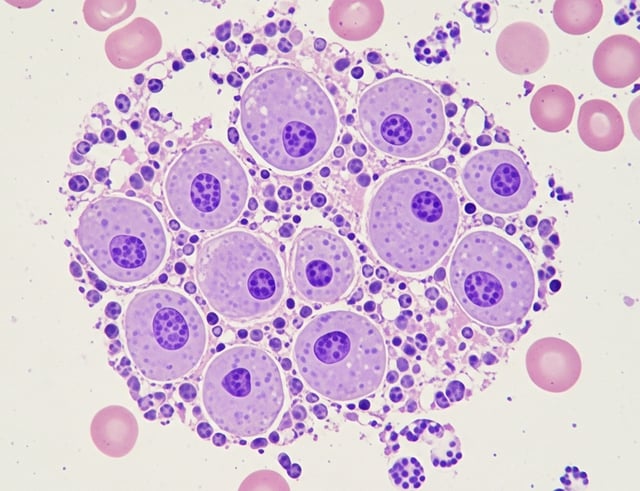
ESO-T01 has therapeutic potential for multiple myeloma
ILLUSTRATION: AI
To solve this problem, the research team has implemented a method called “CAR-T in-vivo” - considered a new breakthrough. Accordingly, instead of operating outside the body, doctors directly infuse a virus carrying “instructions” for producing CAR into the body. T cells in the body will automatically convert into CAR-T, without having to go through a complicated modification or culture process like the traditional method.
The virus being tested is called ESO-T01, a lentivirus specifically designed to target T cells in the body. The virus carries genetic code that creates a CAR against BCMA, a protein commonly found on multiple myeloma cells. Tests in mice showed that ESO-T01 was effective and safe.
The tumor improved significantly after 28 days.
The first clinical trial was conducted on four patients who were being treated at a hospital in China from November 2024 to January 2025. They all had advanced multiple myeloma, which has spread outside the bone, and which is often very difficult to treat. They had already received at least two treatments but the disease still progressed. Some patients were even severely refractory, having failed previous CAR-T therapy.
All patients in the trial received a single intravenous dose of ESO-T01, without prior cell collection or preparatory chemotherapy. They were then monitored for 24 hours with an electrocardiogram and isolated for 48 hours to ensure their safety.

These initial results are very encouraging. However, this is a single-group, open-label, uncontrolled study.
ILLUSTRATION: AI
After receiving ESO-T01, all 4 patients showed symptoms of acute inflammation with similar progression: chills, fever lasting from 6-18 hours. Some other symptoms such as hypotension, hypoxemia, confusion, headache... also occurred in 4 patients in the following days; some were severe, some were mild, but all were closely monitored and controlled promptly, not life-threatening.
As of April 1, 2025, four patients have completed at least two months of follow-up, with the first two patients having completed three months of follow-up. The results show that the four patients' conditions have significantly improved.
Specifically, the intra- and extra-skeletal lesions of 2 out of 4 patients disappeared after 2 months. Notably, patient 2 achieved this improvement after only 28 days. Patients 3 and 4 had significantly reduced tumor size, and bone marrow was free of disease (MRD negative) after 28 days.
According to a research report published in the British medical journal Lancet , CAR-T cells began to appear in the blood from day 4-8, peaking on day 10-17.
According to the study's evaluation, ESO-T01 has high therapeutic potential for refractory multiple myeloma, and is a new technology and breakthrough with great promise.
These initial results are encouraging. However, this is a single-group, open-label, uncontrolled study. Therefore, further studies with larger numbers, longer follow-up periods, and randomized controlled designs are needed to confirm long-term efficacy and safety.
Source: https://thanhnien.vn/loai-virus-moi-giup-dieu-tri-ung-thu-chi-sau-28-ngay-185250720173454242.htm



![[Photo] Award Ceremony of the Political Contest on Protecting the Party's Ideological Foundation](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/22/1761151665557_giaia-jpg.webp)

![[Photo] Comrade Nguyen Duy Ngoc visited and worked at SITRA Innovation Fund and ICEYE Space Technology Company](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/23/1761174470916_dcngoc1-jpg.webp)
![[Photo] General Secretary To Lam and his wife begin their official visit to Bulgaria](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/23/1761174468226_tbtpn5-jpg.webp)
![[Photo] Da Nang: Shock forces protect people's lives and property from natural disasters](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/22/1761145662726_ndo_tr_z7144555003331-7912dd3d47479764c3df11043a705f22-3095-jpg.webp)
![[Infographic] 8 cancers related to tobacco](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/10/18/1760748667221_anh-chup-man-hinh-2025-09-26-110-2182-jpg.webp)





























































































Comment (0)