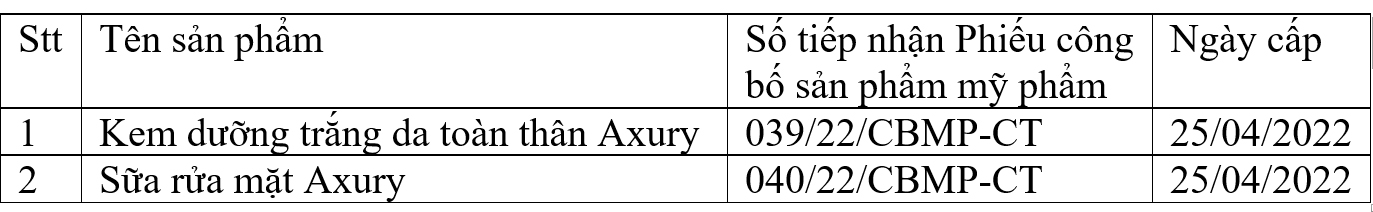
The notice clearly states: Pursuant to Official Dispatch No. 1811/QLD-MP dated February 28, 2023 of the Drug Administration Department (Ministry of Health) on the suspension of circulation and nationwide recall of 02 cosmetic products manufactured by Nguyen Hoang Na Production - Trading One Member Co., Ltd. (Address: No. 2/79B Mau Than, An Hoa Ward, Ninh Kieu District, Can Tho City), Tien Quyen Company Limited (Address: Group 5, Village 3, Ham Liem Commune, Ham Thuan Bac District, Binh Thuan Province) is the one who announced and is responsible for bringing the product to the market as follows:
Reason for suspension of circulation and recall: Cosmetic products manufactured at facilities that do not meet the requirements on personnel, production facilities, and quality management systems as prescribed in Decree 93/2016/ND-CP dated July 1, 2016 of the Government regulating the conditions for cosmetic production.
Department of Health requires:
- Pharmaceutical companies, pharmacies and cosmetic businesses must recall the above cosmetics and report the results to the Department of Health before March 10, 2023. Keep records of cosmetic recalls according to regulations.
- The Health Inspectorate organizes inspections and handles units and facilities that do not recall products according to this document.
- Assign the Planning, Operations, and Finance Department (Pharmaceutical Department) to inspect and supervise; propose handling of units and facilities that do not report on recalls.
We respectfully request the People's Committees of districts and cities to deploy the above content to pharmacies and cosmetic businesses located in their management areas; receive and report the recall results, and at the same time direct the inspection, examination and handling of violating establishments according to current regulations of the Department of Health before March 10, 2023.
NT
Source link





































































































Comment (0)