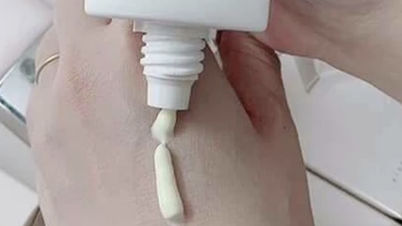
The Drug Administration of Vietnam ( Ministry of Health ) has signed and issued a notice to suspend circulation and recall nationwide three products under the Vinamake brand, including: DR.Hair Ginger Shampoo (registration number: 24/24/CBMP-HB), DR. Ginger Shampoo (registration number: 23/24/CBMP-HB) and nano silver feminine hygiene solution with perfume scent (registration number: 122/23/CBMP-HB).
 |
| Illustration photo. |
All three products are manufactured and marketed by Vinamake Joint Stock Company, located at Group 8, Le Thanh Tong Street, Hoa Binh Ward, Phu Tho Province.
Conclusion from the Institute of Criminal Science ( Ministry of Public Security ) determined that the above products do not contain some ingredients as declared on the label and packaging. The Economic Police Department, Phu Tho Provincial Police also concluded that these are counterfeit goods, seriously violating regulations on the production and circulation of cosmetics.
In response to the incident, the Drug Administration of Vietnam requested Vinamake Company to receive all recalled products and submit a recall report before November 1, 2025.
The Department also recommends that people do not trade, distribute or use infringing products. In case of discovering products on the market, consumers should proactively return them to the supplier and promptly notify the authorities.
In recent times, authorities have continuously discovered many cases related to fake milk, fake medicine, health protection foods and poor quality cosmetics.
Experts warn that fake cosmetics of unknown origin can cause many serious side effects such as irritation, allergies, dermatitis, and even long-term effects on the user's health. Consumers need to be more vigilant, only buy products at reputable establishments, and carefully check the origin, ingredients, and published information before use.
Regarding cosmetics management, the Ministry of Health is soliciting comments on the Draft Decree on cosmetics management, replacing Decree No. 93/2016/ND-CP.
According to the Draft, cosmetic products circulating on the market must ensure that they do not cause harm to human health when used in accordance with instructions, label information, and dosage form. The owner or manufacturer must assess the safety of each product according to the ASEAN Cosmetic Safety Assessment Guidelines.
In addition, cosmetics must meet the requirements for limits on heavy metals, microorganisms and trace impurities as prescribed in the updated appendices from the ASEAN Cosmetic Council (ACC). The Ministry of Health will also publicize the list of ingredients that are banned or have limited concentrations, contents and scope of use for businesses and localities to be aware of.
A notable new point in the Draft is that cosmetic businesses are fully responsible for the content of product advertisements, without having to carry out confirmation procedures with the management agency. However, the advertising content must be consistent with the nature of the product, true to the declared uses, and not misleading as a medicine or capable of treating diseases.
The Ministry of Health proposes to absolutely prohibit acts that take advantage of the reputation of the health sector, such as using images, names, articles, and uniforms of doctors, pharmacists, medical staff, or medical facilities for advertising. At the same time, strictly prohibit the use of misleading language, exaggerating the effects, or making absolute assertions.
Specifically, phrases such as “treatment”, “cure”, “immediate reduction”, “complete cure”, “eradicate”, “100% effective”, “only”, “best”, “top”, “guaranteed”, “absolutely safe” must not be used...
In addition, words related to unpublished uses such as “hair growth stimulant”, “fat reduction”, “weight loss”, “hair growth prevention”, “perspiration cessation” or “permanent tattoo” are also prohibited. All equivalent words, which are on the restricted list of the Draft, are also not allowed to appear in cosmetic advertisements.
In addition to advertising content, the Ministry of Health clearly stipulates the conditions for granting certificates of eligibility for cosmetic production. The production facility must have a team of staff trained in CGMP (ASEAN Cosmetic Good Manufacturing Practices), with sufficient experience; appropriate factory, equipment and quality management system. The person in charge of production and quality must have a university degree in a relevant major, work full-time and have at least 2 years of experience.
The draft Decree, when completed and promulgated, will create a transparent and modern legal corridor, approaching regional standards, contributing to protecting consumer health, improving advertising activities, and improving the quality of the cosmetics market in Vietnam.
Source: https://baodautu.vn/phat-hien-dau-goi-dung-dich-ve-sinh-phu-nu-gia-mang-nhan-hieu-vinamake-d406177.html


![[Photo] Unique Phu Gia horse hat weaving craft](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/10/1760084018320_ndo_br_01-jpg.webp)



![[Photo] Opening of the World Cultural Festival in Hanoi](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/10/1760113426728_ndo_br_lehoi-khaimac-jpg.webp)
![[Photo] Ho Chi Minh City is brilliant with flags and flowers on the eve of the 1st Party Congress, term 2025-2030](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/10/1760102923219_ndo_br_thiet-ke-chua-co-ten-43-png.webp)


























































































Comment (0)