According to the Drug Administration of Vietnam (Ministry of Health), Femancia is produced by Me Di Sun Pharmaceutical Joint Stock Company (An Loi Quarter, Hoa Loi Ward, Ben Cat Town, Binh Duong Province (old), now An Loi Street, Hoa Loi Ward, Ho Chi Minh City).
Recently, Femancia has been prescribed by many medical facilities to treat patients with anemia due to iron deficiency or folic acid deficiency and is sold at many pharmacies nationwide.
In addition to being indicated in cases of iron deficiency anemia, this drug is also used to prevent anemia in subjects with increased iron and folic acid needs such as pregnant women, breastfeeding women, people who have just undergone surgery, and people with serious illnesses who are in the recovery stage.
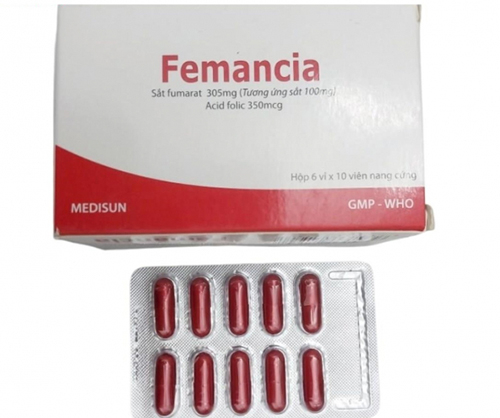
However, through inspection and testing, the authorities discovered that the batch of Femancia medicine, registration number VD-27929-17, dosage form, hard capsule, elemental iron (in the form of ferrous fumarate 305 mg) 100 mg, Folic acid 350mcg violated level 2.
To ensure safety for users, the Drug Administration of Vietnam ( Ministry of Health ) has announced a mandatory recall of this batch of medicine to all businesses, medical facilities and users.
According to Circular 11/2018/TT-BYT of the Minister of Health regulating the quality of drugs and drug ingredients, level 2 drugs are drugs with evidence of not ensuring full treatment effectiveness or posing a risk of being unsafe for users but not to the extent of causing serious harm to health, or not affecting the life of the user.
The Drug Administration of Vietnam requires drug trading and usage establishments, medical examination and treatment establishments not to trade, supply, dispense, or use Femancia and quarantine any remaining drugs at the establishments, and return the drugs to the supplying establishments according to regulations.
Ho Chi Minh City Department of Health inspects and supervises Me Di Sun Pharmaceutical Joint Stock Company to recall and handle the above-mentioned recalled Femancia drug according to regulations; at the same time, evaluate the effectiveness of the drug recall (is the recall thorough, is the product still likely to continue to be circulated, used and has the risk of adversely affecting the health of users or not).
In addition to withdrawing the drug's registration from the list of drugs granted a registration certificate for circulation in Vietnam, the Drug Administration of Vietnam said that Femanica will not be produced and circulated on the market from July 16.
In recent times, in the fight against counterfeiting in the medical field, many types of drugs that violate quality, even counterfeit drugs, have been discovered. According to the Drug Administration of Vietnam, in June, the unit inspected 38 facilities, of which 17 were found to be in violation, including violations of GMP principles, drug quality, and storage conditions.
The Department has issued decisions to temporarily suspend production, request the destruction of substandard drug batches, recall and temporarily stop receiving applications from a number of companies.
Source: https://cand.com.vn/y-te/vi-sao-thuoc-dieu-tri-thieu-mau-femancia-bi-cam-san-xuat-luu-hanh--i775163/




























































































![[Infographic] In 2025, 47 products will achieve national OCOP](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/7/16/5d672398b0744db3ab920e05db8e5b7d)





Comment (0)