On July 23, the Drug Administration of Vietnam ( Ministry of Health ) informed that it had sent a document to the Department of Health of provinces and cities to announce the suspension of circulation and recall and destruction nationwide of 8 batches of facial cleanser and skin care products under the Image and Image Skincare brands distributed by Minh Khuong Company (produced by Image International Manufacturing, LLC) which were found to have incorrect ingredients and formulas on the main label as in the product declaration file.
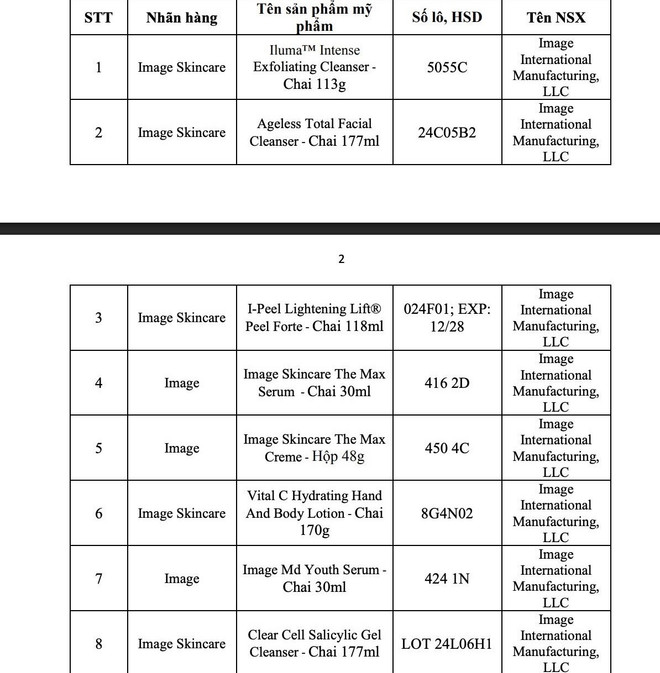
Also related to the above company, the Drug Administration Department also announced the suspension of circulation and nationwide recall of 5 batches of cosmetic products that this company was responsible for bringing to the market.
According to Mr. Ta Manh Hung - Deputy Director of the Department of Drug Administration, the reason for the recall is that the labels of cosmetic products use phrases that cause confusion that the product is a drug.
The Drug Administration of Vietnam requests the Department of Health of provinces and centrally-run cities to notify cosmetic businesses and users in the area to immediately stop selling and using the 13 batches of cosmetic products mentioned above and return them to the product suppliers; recall the above-mentioned violating products; inspect and supervise units implementing this notice; and handle violators according to current regulations.
Minh Khuong Company must send recall notices to distributors and users of the 13 cosmetic product batches mentioned above; receive returned products from business establishments and recall product batches that do not meet regulations.
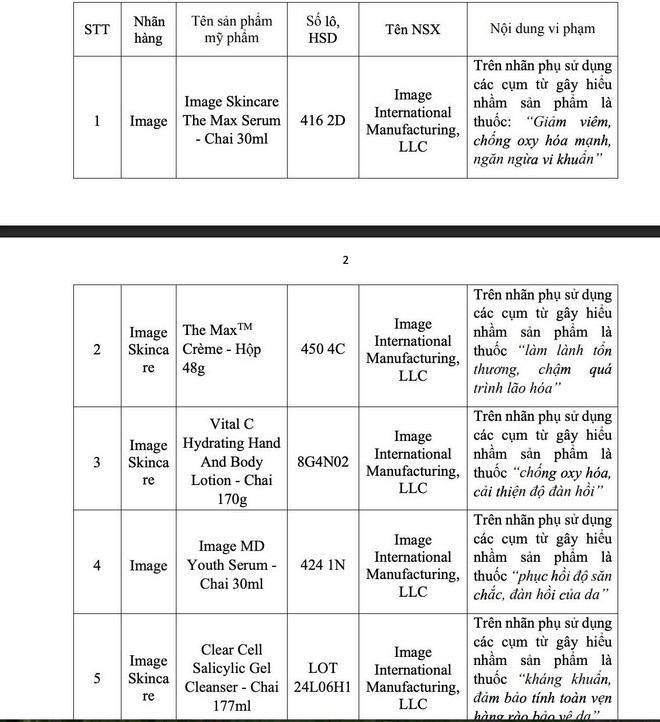
As for the 5 batches of products that are misleading as drugs, in case the violating elements cannot be removed (the violating product label cannot be separated from the goods), the above products must be destroyed according to the provisions of Clause 7, Article 67 of Decree No. 117/2020/ND-CP).
The Drug Administration also requested relevant units to review the cosmetic product labels for other imported batches of violating products. In case of similar errors as the above-mentioned violating batch, the Company must promptly notify the distribution and usage establishments of the recall and send a report on the review and recall of the product to the Drug Administration before July 29, 2025.
The Drug Administration of Vietnam stated that Minh Khuong Trading Company Limited is fully responsible before the law if the report is not truthful.
The Drug Administration also requested the Ho Chi Minh City Department of Health to supervise Minh Khuong Trading Company Limited in recalling 05 cosmetic products that do not meet regulations; Report the supervision results to the Drug Administration before August 29, 2025./.
Source: https://www.vietnamplus.vn/bo-y-te-yeu-cau-thu-hoi-tieu-huy-nhieu-lo-sua-rua-mat-cham-soc-da-post1051222.vnp







































































































Comment (0)