According to Professor Dr. Le Van Quang, Director of K Hospital, the unit will soon introduce Pembroria medicine in the treatment regimen for cancer patients. The drug Pembroria (Russia) is produced, costs about 18 million VND per bottle, patients usually use two bottles in a course. The course of treatment lasts from 12 to 24 courses, or until the patient no longer responds to the drug. Currently, the drug is not covered by health insurance.
According to the representative of the Drug Administration ( Ministry of Health ), Pembroria has completed clinical studies and has been officially licensed for circulation in Vietnam. The drug is produced by Biocad (Russia), registered by a company headquartered in the UAE. The license allows the drug to be imported, distributed and widely used, and is not in the group of special or restricted drugs.
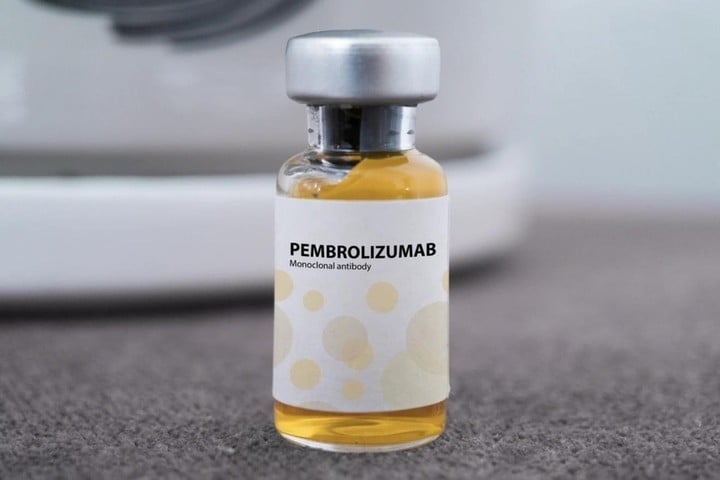
In essence, Pembroria is a “biosimilar” of Keytruda - the original product developed by MSD (USA), which also contains the active ingredient Pembrolizumab. This is a monoclonal antibody that helps the immune system recognize and destroy cancer cells, and is considered one of the most effective immunotherapies today. Keytruda was approved by the US in 2014 and licensed for circulation in Vietnam in 2017.
The appearance of Pembroria is expected to open up more access to treatment for cancer patients, when the cost is significantly lower – about 18 million VND per vial, compared to 55-65 million VND for Keytruda. However, both of these drugs are still not covered by health insurance.
Pembrolizumab works by “rapid release” to T immune cells. Normally, cancer cells sends a “stop” signal that prevents T cells from attacking. The drug will block this signal, reactivating the ability to destroy tumors, effective on many types of cancer such as lung, kidney, cervical, melanoma...
According to the Drug Administration, the entire drug circulation license dossier was submitted by the enterprise in accordance with regulations. By 2024, this drug had completed clinical studies. However, the enterprise continued to conduct an immunogenicity assessment - a mandatory requirement for biosimilar drugs like this product, not a new clinical trial.
During the circulation process, enterprises are responsible for monitoring and periodically reporting on the safety, effectiveness and immunogenicity of the drug after 3 or 5 years, as required by the management agency.
Previously, many drugs containing the active ingredient Pembrolizumab were licensed for circulation in the country. Decision 628/QD-QLD dated October 31 of the Drug Administration approved 14 vaccines and biological products, including Pembroria (100mg/4ml), concentrated solution for infusion, with an expiry date of 24 months from the date of manufacture.
Source: https://baolangson.vn/thuoc-ung-thu-pembroria-cua-nga-vua-duoc-cap-phep-tai-viet-nam-co-gia-bao-nhieu-5064810.html


![[Photo] The "scars" of Da Nang's mountains and forests after storms and floods](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1762996564834_sl8-jpg.webp)
![[Photo] General Secretary To Lam visits Long Thanh International Airport Project](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1763008564398_vna-potal-tong-bi-thu-to-lam-tham-du-an-cang-hang-khong-quoc-te-long-thanh-8404600-1261-jpg.webp)























































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)







Comment (0)