The Drug Administration of Vietnam, Ministry of Health has recently licensed the circulation and use of the drug Pembroria (main active ingredient is Pembrolizumab, content 100mg/4ml) produced by Limited Liability Company "PK-137" (Russia).
This is the next targeted cancer drug approved for use in Vietnam, helping patients access immunotherapy at an affordable cost.
Previously, Vietnam also approved the use of the Irish-made drug Keytruda in cancer treatment from 2021. This drug also has the main active ingredient Pembrolizumab and a content of 100mg/4ml similar to Pembroria.
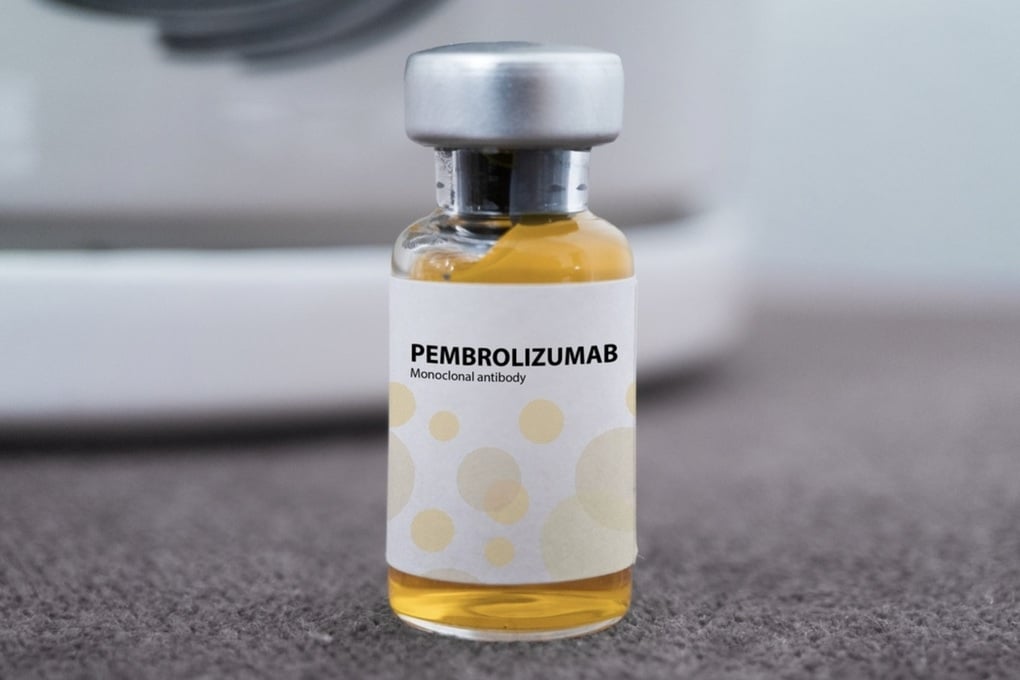
Pembrolizumab is the main active ingredient in the cancer drugs Pembroria and Keytruda licensed in Vietnam (Photo: incentra).
What is immunotherapy?
Immunotherapy is a cancer treatment that encourages the body's immune system to seek out and attack cancer cells. It differs from traditional chemotherapy, which works by directly attacking cancer cells.
The immune system is the body's natural defense against disease and infection. The immune system uses T cells - white blood cells. These cells work by detecting and destroying foreign cells in the body, such as cancer cells. Cancer cells and T cells communicate with each other through a "gate" called the PD-1/PD-L1 pathway.
The PD-1/PD-L1 pathway helps the immune system stay balanced, like having both a gas pedal and a brake pedal. However, cancer cells take advantage of this mechanism to constantly "pull the brakes", causing the immune system to stop working. Specifically, cancer cells express the PD-L1 protein on their surface. This protein will connect to the PD-1 protein on T cells.
When PD-L1 (on cancer cells) and PD-1 (on T cells) bind, the T cells are prevented from killing the cancer cells. This allows the cancer to grow freely.
Immunotherapies like Pembrolizumab are designed to block this connection, thereby releasing the brakes and reactivating the immune system, allowing T cells to kill cancer.
How does pembrolizumab work?
Pembrolizumab (the active ingredient in Pembroria) is a monoclonal antibody. This is a protein made in the laboratory to mimic the body's natural antibodies.
Pembrolizumab works by blocking the protein PD-1 on the surface of T cells. By blocking PD-1, it prevents the connection between PD-1 and PD-L1. This blocking acts as a "brake" on the immune system, making it easier for T cells to recognize and kill cancer cells.
According to the Cancer Prevention Research Institute, Pembrolizumab has been shown to be effective in treating many types of cancer.
The KEYNOTE-001 study, published in the New England Journal of Medicine in 2015, showed that Pembrolizumab improved survival in patients with non-small cell lung cancer.
Specifically, the study focused on late-stage patients and showed that Pembrolizumab improved survival compared to conventional chemotherapy. This effect was particularly pronounced in patients with high PD-L1 expression levels (over 50%).
In addition, the KEYNOTE-240 study published in Lancet Oncology in 2020 also demonstrated the effectiveness of Pembrolizumab in the treatment of inoperable liver cancer.
Despite not meeting the primary statistical endpoints, patients receiving Pembrolizumab still showed improvements in survival and response.
What types of cancer is Pembroria indicated for?
According to the instructions for use from the marketing authorization, Pembroria is indicated for many types of cancer.
These include: melanoma; non-small cell lung cancer; squamous cell carcinoma of the head and neck; classical Hodgkin lymphoma; urothelial cancer; esophageal cancer; colorectal cancer; non-colorectal cancer; cervical and endometrial cancer; renal cell carcinoma; triple-negative breast cancer; adenocarcinoma of the stomach or gastroesophageal junction; and cholangiocarcinoma.
Depending on the type of cancer and stage of the disease, the drug will be prescribed in different doses and methods, and can be combined with chemotherapy.
This medicine is used in both outpatient and hospital settings. Treatment must be initiated and supervised by experienced oncologists.
To be prescribed the above drug, patients must perform PD-L1 testing (immunohistochemistry testing to measure the amount of PD-L1 protein on the surface of cancer cells), testing to determine the tumor status of microsatellite instability (MSI) or mismatch repair (MMR) deficiency.
Source: https://dantri.com.vn/suc-khoe/co-gi-trong-thuoc-pembroria-chua-ung-thu-vua-duoc-cap-phep-hoat-dong-20251112144021636.htm




![[Photo] General Secretary To Lam visits Long Thanh International Airport Project](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1763008564398_vna-potal-tong-bi-thu-to-lam-tham-du-an-cang-hang-khong-quoc-te-long-thanh-8404600-1261-jpg.webp)

![[Photo] The "scars" of Da Nang's mountains and forests after storms and floods](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1762996564834_sl8-jpg.webp)























































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)








Comment (0)