On November 21, the Department of Food Safety (Ministry of Health) sent a document to the Department of E-commerce and Digital Economy ( Ministry of Industry and Trade ) regarding the management of functional food business on e-commerce.
According to the Food Safety Administration, on November 19, the international food safety agency system (INFOSAN) announced an ongoing case of infant botulism poisoning in the United States related to the consumption of contaminated ByHeart Whole Nutrition Infant Formula.
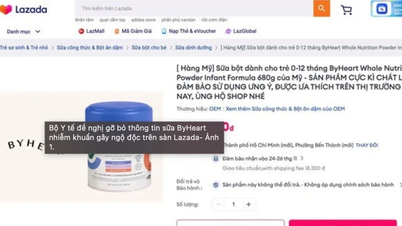
Currently, on some e-commerce platforms and e-commerce applications in Vietnam such as Lazada... there is a sale of ByHeart Whole Nutrition Infant Formula milk formula products.
Therefore, the Food Safety Department requested the Department of E-commerce and Digital Economy to request e-commerce platforms and e-commerce applications to remove the above product information to ensure consumer health and resolve customer complaints and suggestions (if any).
As of November 17, 2025, the US Centers for Disease Control and Prevention (CDC) has reported 23 suspected and confirmed cases across 13 states, all of whom have required hospitalization.
Parents and caregivers in the United States are advised to immediately stop using any ByHeart Whole Nutrition Infant Formula products, including all lot numbers, all can sizes, and single-serve packages.
Currently, ByHeart Inc, USA (manufacturer) is voluntarily recalling all batches of ByHeart Whole Nutrition Infant Formula on the market.
To ensure the health of consumers, the Department of Food Safety has issued Official Dispatch No. 2347/ATTP-SP dated November 20, 2025 to the Department of Health , Department of Food Safety, and Food Safety Sub-Departments of provinces and cities to urgently deploy a number of contents, including reviewing the registration of declarations and self-declarations for the formula milk product ByHeart Whole Nutrition Infant Formula produced by ByHeart Inc., USA.
Relevant units work with the company that announced the ByHeart Whole Nutrition Infant Formula product (if any), requesting the company to notify distributors and consumers to stop using the product and recall it according to the manufacturer's recommendations, report (quantity imported, quantity sold, quantity remaining) and propose measures to handle these product batches./.
Source: https://www.vietnamplus.vn/bo-y-te-de-nghi-go-bo-thong-tin-sua-byheart-gay-ngo-doc-tren-san-thuong-mai-dien-tu-post1078530.vnp











































































































Comment (0)