
The leaders of K Hospital said they are developing a plan to purchase Pembroria, with an estimated cost of about 36 million VND for each treatment cycle of a patient. In the South, representatives of Cho Ray Hospital and Dr. Diep Bao Tuan, Director of Ho Chi Minh City Oncology Hospital, said they will organize a public bidding to purchase Pembroria according to regulations. If the drug is licensed and wins the bid, hospitals will use it for patients according to professional instructions.
Pembroria medicine with the active ingredient pembrolizumab, 100mg/4ml vial, manufactured by Limited Liability Company "PK-137", Russia, has been licensed by the Ministry of Health for circulation in Vietnam since the end of October. Pembrolizumab is the name of the active ingredient (active ingredient) invented by the American company MSD with the trade name Keytruda, 100mg/4ml vial. Pembrolizumab is essentially an anti-PD-1 monoclonal antibody and is a biological drug indicated for certain types of cancer, belonging to the immunotherapy group. The drug is administered by intravenous infusion at a medical facility.
The question that many people ask is how effective the drug is and what the treatment regimen and protocol are. Currently, the Ministry of Health has not announced the treatment effectiveness of Pembroria based on the results of the testing phases according to the application for a marketing license. However, doctors say that the effectiveness and treatment regimen depend on each patient's condition.
Explaining this, according to Dr. Le Tuan Anh, Director of the Oncology Center, Cho Ray Hospital, in cancer treatment, the treatment regimen is built based on individualization for each type of disease, each specific stage of each patient, not simply based on a single drug. The doctor at K Hospital also said that the use of pembrolizumab also depends on many factors such as the patient's condition, the type of tumor mutation, the stage of the disease... assessed by oncologists.
Compared to Pembroria, Keytruda was approved by the US nearly 10 years ago, so it is currently used in many cancer treatment facilities in the country and is the best-selling cancer drug in the world today. At Cho Ray Hospital, nearly 500 patients have accessed the American drug in the past 5 years, mainly lung cancer, head and neck cancer, melanoma... with treatment results that are evaluated positively.
Like Keytruda, Pembroria is a biologic drug with the same active ingredient, so it is approved for a very broad spectrum of indications. The drug is used to treat cases of melanoma, non-small cell lung carcinoma, head and neck squamous cell carcinoma, classical Hodgkin lymphoma, urothelial carcinoma, esophageal carcinoma, cancers with high-grade microsatellite instability (MSI-H) or mismatch repair defects, cervical cancer, renal cell carcinoma, endometrial carcinoma, triple-negative breast cancer, adenocarcinoma of the stomach or gastroesophageal junction, and cholangiocarcinoma.
However, "not all patients with the cancers listed above are prescribed pembrolizumab," said the leader of K Hospital, adding that treatment indications will be individualized for each patient. Based on many factors, doctors will advise and provide appropriate treatment regimens to ensure effective treatment for patients.
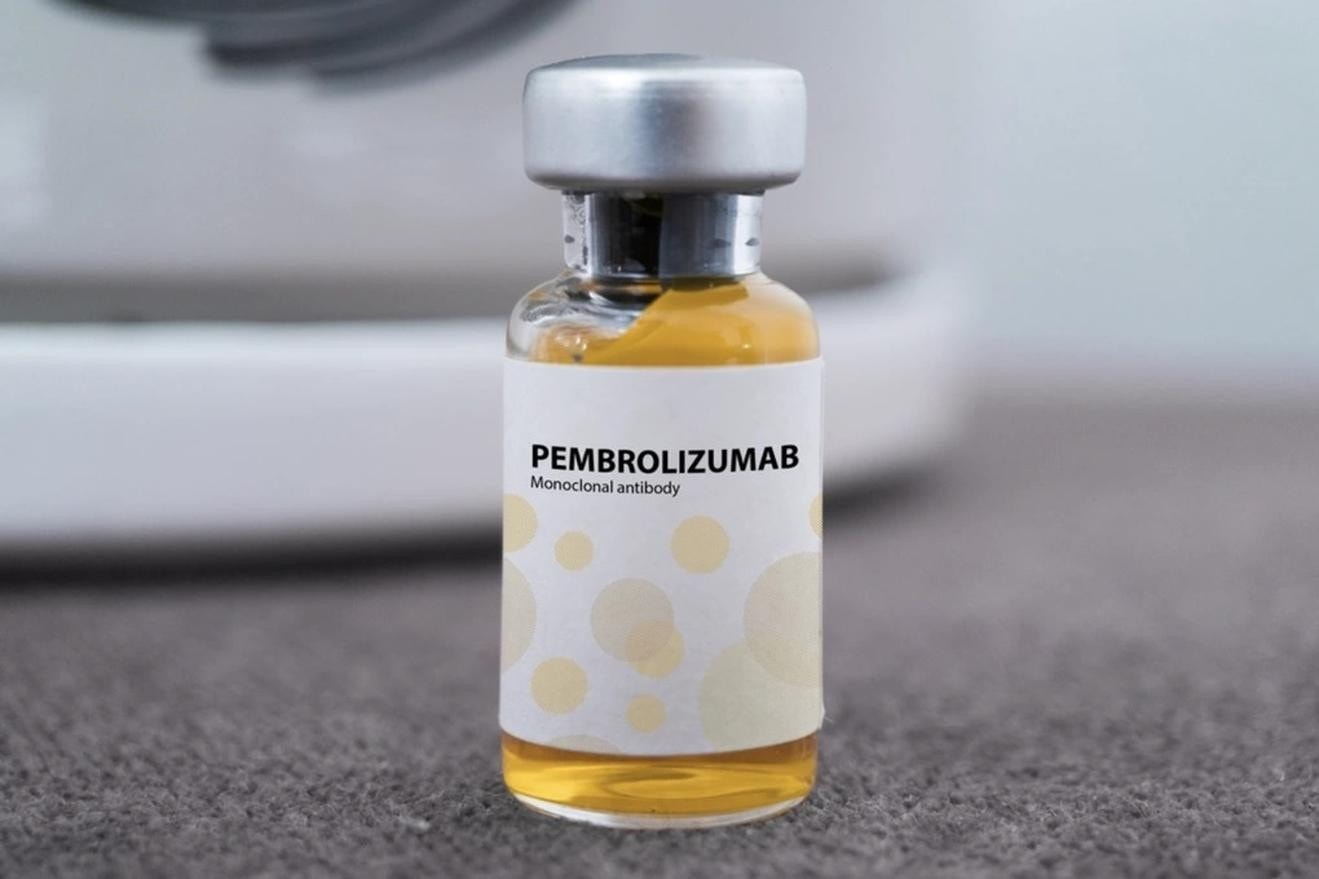
Another issue of concern is the price of the drug and the cost of treatment. According to doctors, the expected price of Pembroria is about 18 million VND/bottle, with the usual dose being two bottles for a treatment cycle. Thus, the cost for a patient's treatment cycle is about 36 million VND with a dose of 200 mg (two bottles) - as calculated by the leaders of K Hospital to develop a drug purchasing plan.
Thus, compared to the original drug Keytruda, Pembroria has a lower price, only 1/3. The current price of Keytruda is about 62 million VND/bottle. However, Keytruda has a partial free support program for eligible cancer patients of the Ministry of Health, which is being implemented at K Hospital and many other hospitals nationwide. With this program, the maximum cost for a patient's treatment cycle is about 62 million VND with a dose of 200 mg (two bottles) and some cycles can be completely free, depending on the level of support that the patient is approved for.
These programs, approved by the Ministry of Health and sponsored by pharmaceutical companies, are considered a “bridge financing mechanism” to help patients access new, expensive drugs while waiting for health insurance coverage. This helps reduce the burden of hospital fees and maintain effective treatment regimens. For example, with targeted therapy drugs for cancer patients, the average cost is about one billion VND per year. When participating in the program, patients co-pay 30-40%. The level of support increases gradually over the treatment period.
According to statistics from the Department of Health Insurance (Ministry of Health), in the period 2019-2024, programs like this supported 10,652 patients, mainly those with cancer, hematological diseases, psoriasis, arthritis and retinal diseases. The common form of support is co-payment, helping patients reduce financial pressure so they do not have to abandon their treatment regimen.
According to doctors, targeted drugs and immunotherapy are expensive, not covered by health insurance, and patients mainly pay for them themselves, which is a burden for patients and their families. A person treated with targeted drugs or immunotherapy can spend 120-150 million VND per month, about 500-600 million VND to several billion VND per year, depending on the indication and type of drug. Each patient may have to be treated for one to two years. Currently, only about 10% of cancer patients have access to this therapy.
At K Hospital, the average cost of treatment for a cancer patient is over 176 million VND per year. Insurance covers about 52 million VND and the patient must pay about 124 million VND, accounting for 70% of the total cost of treatment.
According to VnESource: https://baohaiphong.vn/cac-benh-vien-len-ke-hoach-mua-thuoc-ung-thu-cua-nga-526727.html



![[Photo] Action for the Community tells stories of enduring journeys – both intimate and great, yet quiet and determined](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/15/1763179022035_ai-dai-dieu-5828-jpg.webp)







































































































Comment (0)