According to the representative of the Drug Administration, the issuance of the decision to license the drug Pembroria for cancer treatment is completely in accordance with current procedures and regulations. This drug has completed clinical trials and been approved by the scientific council.
Before being granted a registration number for circulation in Vietnam, the product profile was evaluated by pharmacologists of Hanoi Medical University and confirmed to have met the requirements for effectiveness and immunogenicity similar to the original drug. Furthermore, Pembroria is licensed for widespread circulation in Vietnam like many other drugs, not licensed for clinical trials.

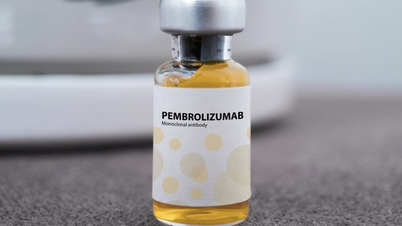
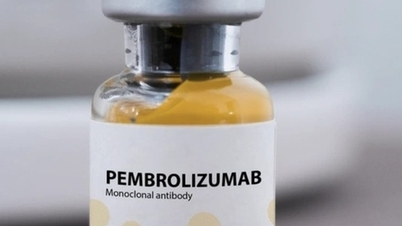
Pembroria cancer treatment drug has just been licensed for circulation.
In the licensing decision, the Drug Administration of Vietnam requires the company, after receiving the circulation registration certificate, to update the progress of phase III clinical research on immunogenicity, report periodically every 3 months and submit updated data when the research is completed.
According to the explanation of the representative of the Drug Administration, although the drug has met the safety and quality standards to be circulated, the manufacturing company will continue to monitor and evaluate the immunogenicity of the drug for Vietnamese people. This is a mandatory requirement for the group of biosimilar drugs because immunogenicity can cause immune reactions in the user's body. Along with that, this requirement is higher than usual, not a product that has not completed clinical trials has been registered for circulation as some opinions are misunderstanding.
According to the records, Pembroria is a biosimilar, or "copy" of the drug Keytruda developed by MSD (USA). Both drugs contain the active ingredient Pembrolizumab, a monoclonal antibody that helps the immune system recognize and attack cancer cells. Keytruda was first approved by the US in 2014 and is currently one of the most popular immunotherapies in cancer treatment in the world .
Pembroria is prepared in the form of a concentrated solution for infusion, with a shelf life of 24 months from the date of manufacture. According to information from the drug registration unit, Pembroria is indicated for the treatment of many types of cancer, such as: lung carcinoma, melanoma, colorectal cancer, cervical cancer, renal cell carcinoma, breast cancer...
The price of Pembroria in Vietnam in the early stages was about 18 million VND per bottle, while the price of Keytruda ranged from 55-65 million VND per bottle.
MINH KHANG
Source: https://www.sggp.org.vn/thong-tin-ve-thuoc-dieu-tri-ung-thu-pembroria-vua-duoc-cap-phep-tai-viet-nam-post823216.html





![[Photo] Deep sea sand deposits, ancient wooden ship An Bang faces the risk of being buried again](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1763033175715_ndo_br_thuyen-1-jpg.webp)































































![[Photo] Panorama of the 2nd Vietnam-Cambodia Border Defense Friendship Exchange](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/13/1763033233033_image.jpeg)


















![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)







Comment (0)