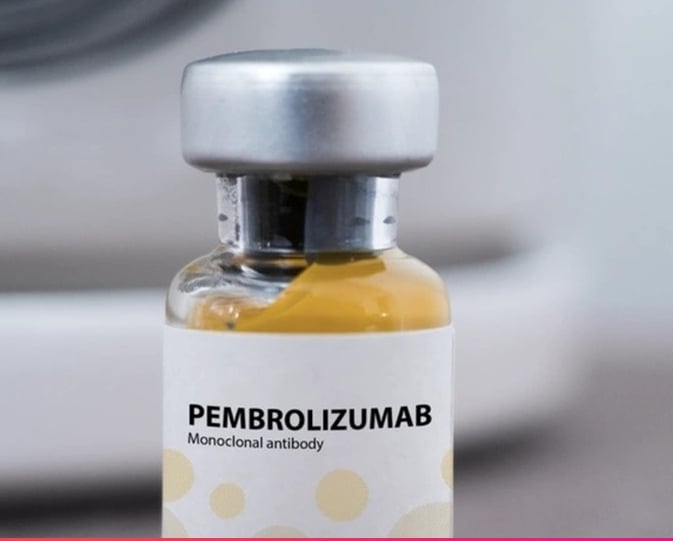
According to K Hospital, Pembrolizumab is the name of the active ingredient (active ingredient) invented by MSD of the United States with the trade name Keytruda, 100mg/4ml bottle. Keytruda has been licensed for circulation in Vietnam by the Drug Administration of Vietnam - Ministry of Health since 2017.
Pembrolizumab is an anti-PD-1 monoclonal antibody and is a biologic drug indicated for certain types of cancer, belonging to the immunotherapy group. The drug is administered by intravenous infusion at a medical facility.
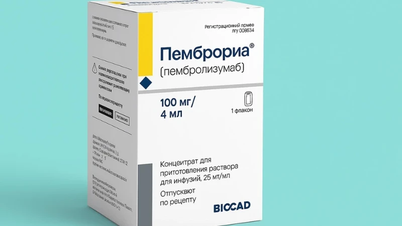
Previously, as Health and Life reported, on October 31, 2025, the Drug Administration of Vietnam - Ministry of Health granted a circulation registration certificate for the drug Pembroria with the active ingredient pembrolizumab, 100mg/4ml vial manufactured by Limited Liability Company "PK-137", Russian Federation.
Pembroria medicine is prepared in the form of a concentrated solution for infusion, with a shelf life of 24 months from the date of manufacture.
The Russian cancer treatment drug Pembroria (active ingredient pembrolizumab) has been granted a circulation registration certificate by the Drug Administration of Vietnam - Ministry of Health. Currently, K Hospital is planning to purchase Pembroria to use for patients in the near future.
Pembroria is a biologic medicine with the same active ingredient as Keytruda, approved for the following indications: Melanoma, Non-small cell lung carcinoma, Head and neck squamous cell carcinoma, Classic Hodgkin lymphoma, Urothelial carcinoma, Esophageal carcinoma, Cancers with microsatellite instability-high (MSI-H) or mismatch repair defects, Cervical cancer, Renal cell carcinoma, Endometrial carcinoma, Triple-negative breast cancer, Adenocarcinoma of the stomach or gastroesophageal junction, Cholangiocarcinoma.
The expected price of Pembroria is about 18 million VND/bottle, with the usual dose being 2 bottles for 1 treatment cycle. Thus, the cost for a patient's treatment cycle is about 36 million VND with a dose of 200mg (2 bottles). Currently, K Hospital is planning to purchase Pembroria for use in patients in the near future.
K Hospital emphasizes: Not all patients with the cancers listed above are prescribed pembrolizumab.
The use of pembrolizumab also depends on many factors such as: the patient's health status, tumor mutation type, disease stage... assessed by oncologists.
At K Hospital, treatment will be individualized for each patient. Based on many factors, the doctor will advise and provide a suitable treatment regimen to ensure effective treatment for the patient.
Regarding the drug Keytruda, K Hospital said that this drug has been approved by the Ministry of Health for 14 indications including: malignant melanoma, non-small cell lung cancer, classical Hodgkin lymphoma, urothelial carcinoma, head and neck squamous cell carcinoma, gastric cancer, cancer with high-level microsatellite instability, cervical cancer, hepatocellular carcinoma, colorectal cancer with high-level microsatellite instability or mismatch repair defect, primary mediastinal large B-cell lymphoma, esophageal cancer, triple-negative breast cancer, renal cell carcinoma.
The current price of Keytruda is about 62 million VND/bottle. The drug has a partial free support program for eligible cancer patients according to Decision No. 2205/QD-BYT dated July 2, 2025 of the Ministry of Health.
The above support program is being implemented at K Hospital and many other hospitals nationwide.
With this program, the maximum cost for a patient's treatment cycle is about 62 million VND with a dose of 200mg (2 bottles) and some cycles can be completely free, depending on the level of support the patient is approved for.
Source: https://suckhoedoisong.vn/benh-vien-k-noi-gi-ve-thuoc-dieu-tri-ung-thu-cua-nga-vua-duoc-cap-luu-hanh-tai-viet-nam-169251113203829912.htm






![[Photo] Deep sea sand deposits, ancient wooden ship An Bang faces the risk of being buried again](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1763033175715_ndo_br_thuyen-1-jpg.webp)


















































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)







Comment (0)