The results were announced at the largest annual meeting of cancer experts, organized by the American Society of Clinical Oncology (ASCO) in Chicago, USA. Lung cancer is the deadliest form of the disease, with about 1.8 million deaths each year worldwide .
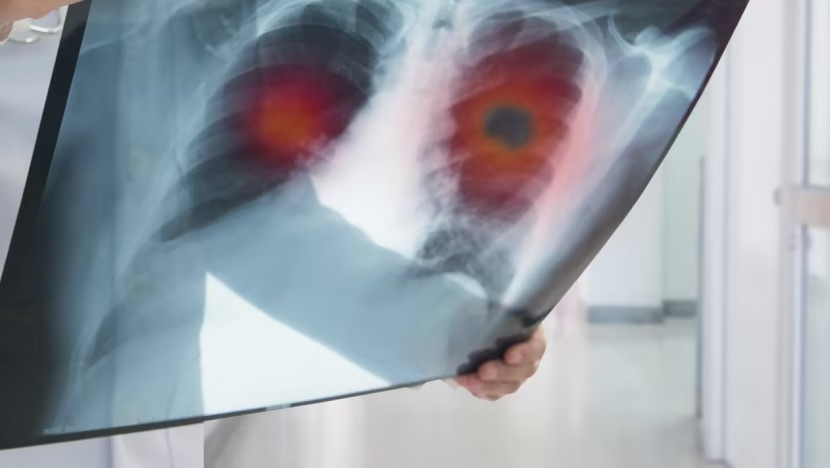
A doctor examines an X-ray image of a lung tumor. Photo: iStock
The treatment, developed by pharmaceutical giant AstraZeneca, is called osimertinib and is marketed as Tagrisso. It targets a type of lung cancer in patients with what is known as non-small cell lung cancer and has a specific mutation.
This is the most common type of lung cancer. These mutations affect 10% to 25% of lung cancer patients in the United States and Europe, and up to 30 to 40% in Asia.
The clinical trial involved 680 participants with early-stage disease (stages 1b to 3a) in more than 20 countries. They first had surgery to remove the tumor, then half of the patients received daily treatment and the rest received placebo therapy (drugs that had no therapeutic effect).
Results showed that those taking the drug had a 51% lower risk of death than those taking the placebo. After five years, 88% of treated patients were still alive, compared with 78% of patients taking the placebo.
The data were “very impressive,” said Yale University’s Roy Herbst, who presented the trial results in Chicago. He added at a news conference that the drug “prevented cancer from spreading to the brain, the liver, the bones.”
Osimertinib has been licensed in dozens of countries for various indications and has been given to about 700,000 people, according to a press release from AstraZeneca.
Herbst said not all doctors are using the treatment, and many are waiting for overall survival data to be released Sunday.
Mai Van (according to AFP, CNA)
Source



















































![[Maritime News] More than 80% of global container shipping capacity is in the hands of MSC and major shipping alliances](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/7/16/6b4d586c984b4cbf8c5680352b9eaeb0)











































Comment (0)