30% of patients are having to pay out of pocket for medical treatment. Along with ensuring supply, drug prices must be tightly controlled with the principle of people accessing new drugs as quickly as possible and at the most suitable price.
Medicines must ensure quality and reasonable price.
"Pharmaceutical business must not be inflated or go around in circles. Business needs to harmonize the interests of enterprises (growth); the state (tax revenue) and the people (access to reasonable prices). Going around in circles to raise prices is very dangerous. If not done properly, it is easy to get into trouble with the law," Deputy Minister of Health Do Xuan Tuyen warned at the conference on pharmaceutical and cosmetic work organized by the Ministry of Health in Quang Ninh province today, December 17.
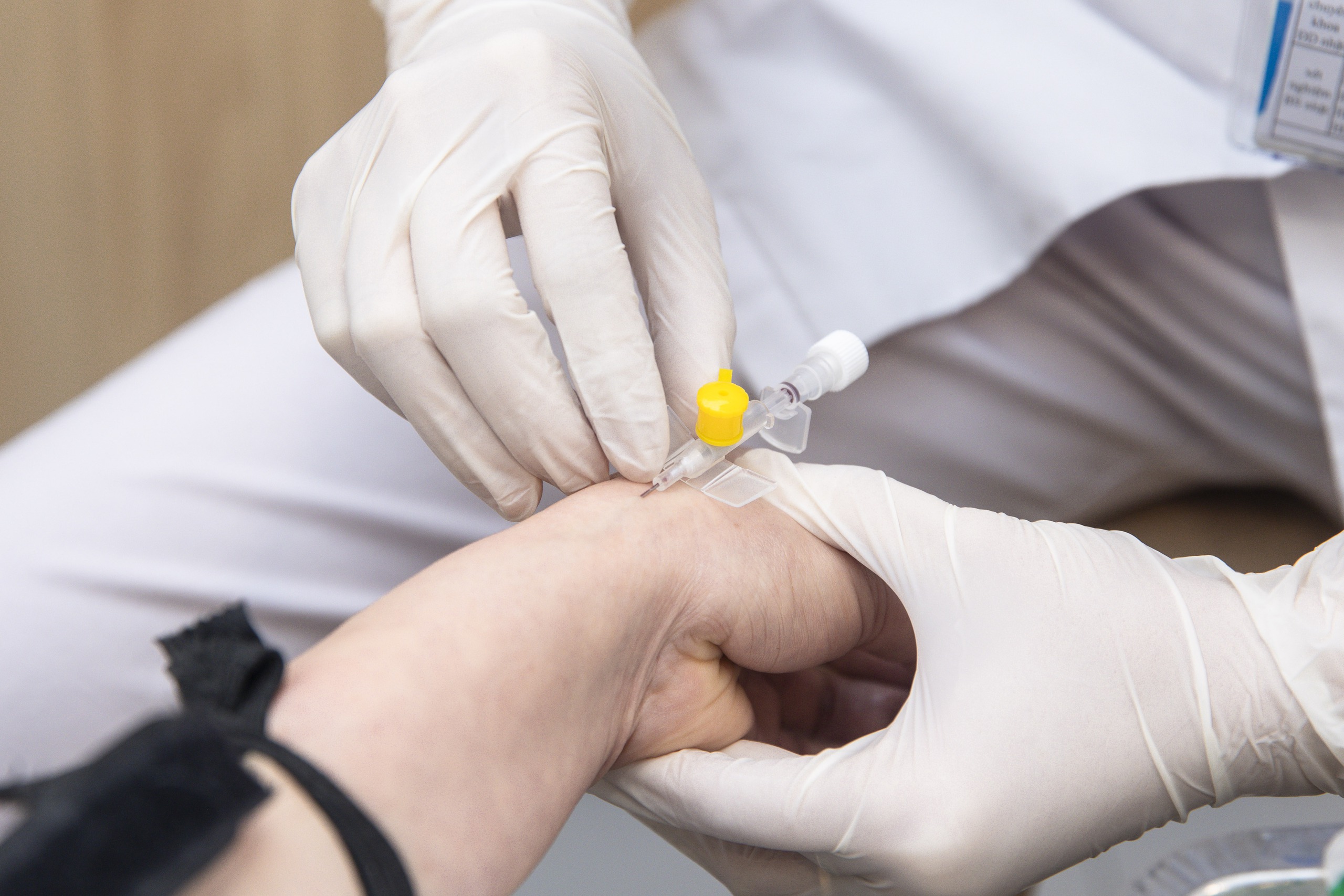
Along with ensuring supply, the Drug Administration strengthens drug quality control.
Along with ensuring supply, Mr. Tuyen emphasized controlling drug prices with the principle of people accessing new drugs as quickly as possible at the most suitable price, in the context that 30% of patients are having to pay out of pocket for medical examination and treatment.
According to Mr. Tuyen, the Pharmaceutical Law, which will take effect from 2025, requires businesses to announce their expected prices. The Drug Administration will conduct post-audit and, if unreasonable, will recommend adjustments; if not, it will be transferred to the competent authority.
No medicine, how to bid?

The Ministry of Health continues to implement solutions to help people access new drugs as quickly as possible at reasonable prices.
Regarding the supply of treatment drugs, Mr. Tuyen noted: "If drugs are not circulated, where will they come from to bid?". According to him, speeding up administrative reform of drug registration and registration renewal procedures is the focus of pharmaceutical management.
Mr. Tuyen acknowledged that about 80 - 90% of raw materials for drug production must be imported, the mechanism is still inadequate, in which some regulations issued by the Drug Administration Department and the Traditional Medicine Administration Department have not met the requirements, there may be "redundant" regulations, with the risk of being sub-licenses.
"Administrative procedures for pharmaceutical work and drug registration have increased dramatically. Before 2020, the Ministry of Health issued more than 100 drug registration numbers each year, in 2022 there were 2,721 records and in 2023 there were 4,592 records. This year alone there were nearly 14,000 records, not to mention the extension of over 13,000 records," Mr. Tuyen said.
Mr. Vu Tuan Cuong, Director of the Department of Drug Administration, said that ensuring medicine for medical examination and treatment depends on the supply source and is related to the implementation of bidding at medical facilities.
In particular, the Drug Administration has overcome procedures to ensure input. There are regularly more than 23,000 drug registration numbers in the country, ensuring supply.
In 2024 alone, there will be a breakthrough in registration number issuance with comprehensive digital transformation, from application submission, to appraisal, and licensing approval council meetings. The Drug Administration strives to issue on time for applications submitted from January 1, 2024, while also resolving the backlog of old applications. In 2023, 75% of applications will be issued on time. From July 1, 2024, all drugs will be issued online registration numbers.
From July 2023, procedures for granting, renewing, changing, and supplementing drug and pharmaceutical ingredient circulation registration certificates have been implemented, received, appraised, and processed online.
In the first 11 months of 2024 alone, the number of drugs (13,164 drugs) granted circulation registration certificates is equal to the total of the previous 5 years.
Source: https://thanhnien.vn/nguoi-dan-can-duoc-tiep-can-thuoc-moi-nhanh-nhat-gia-phu-hop-nhat-185241217090841411.htm







![[Photo] Closing of the 13th Conference of the 13th Party Central Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/08/1759893763535_ndo_br_a3-bnd-2504-jpg.webp)




















































































Comment (0)