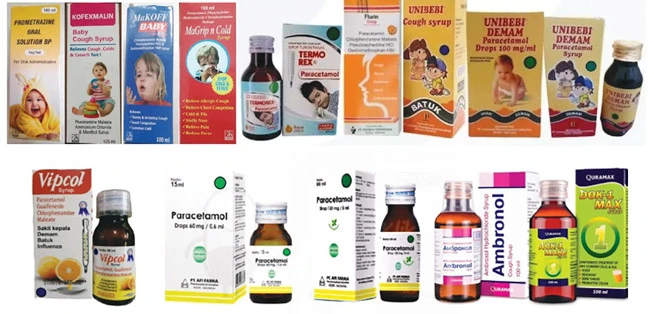
According to the Drug Administration of Vietnam ( Ministry of Health ), through searching the data on circulation registration certificates, including the records being processed at the Department and the data on import licenses for drugs without circulation registration certificates according to the provisions of Decree 54/2017, as of December 13, the products Alergo syrup, Emidone suspension, Mucorid syrup, Ulcofin suspension and Zincell syrup of Pharmix Laboratories (Pakistan) have not been granted circulation registration in Vietnam.
In April this year, the Ministry of Health informed about 14 types of cough medicine containing diethylene causing serious damage to health.
The Drug Administration also determined that Pharmix Laboratories (Pakistan) has not had any drugs registered for circulation in Vietnam and has not submitted any drug registration documents to the Drug Administration, and has not been granted an import license for drugs that do not have a registration certificate for circulation.
Recently, the World Health Organization (WHO) warned that it had discovered that some cough syrups and suspensions with active ingredients to treat certain medical conditions contained levels of ethylene glycol exceeding the permitted level.
WHO stated that the affected products were all manufactured by Pharmix Laboratories (Pakistan) and were first detected in Maldives and Pakistan.
WHO said a total of batches of Alergo syrup, Emidone suspension, Mucorid syrup, Ulcofin suspension and Zincell syrup were affected.
According to the warning, the concentration of ethylene glycol in the drugs ranges from 0.62 - 0.82%, higher than the permitted level of 0.1%. These drugs are used to treat coughs, allergies and other health problems.
WHO warns that substandard and unsafe products; and that their use, especially by children, can lead to health damage.
Pharmax Laboratories has not commented on the above information. WHO has not recorded any cases of side effects due to these drugs.
Earlier, in April this year, the Ministry of Health said it had received a telegram from the International Criminal Police Organization (Interpol) warning about children suffering from acute kidney injury after using 14 syrup products banned in several countries. These products contain diethylene which can lead to serious health damage.
The Drug Administration has requested the health departments of provinces and cities to conduct inspections and checks at pharmaceutical establishments regarding the circulation of these products in particular and drugs of unknown origin and origin, and drugs that have not been licensed for circulation in general on the market.
Source link





















































































![[Infographic] In 2025, 47 products will achieve national OCOP](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/7/16/5d672398b0744db3ab920e05db8e5b7d)













Comment (0)