Recall of popular drug batch for treating swelling after trauma and burns
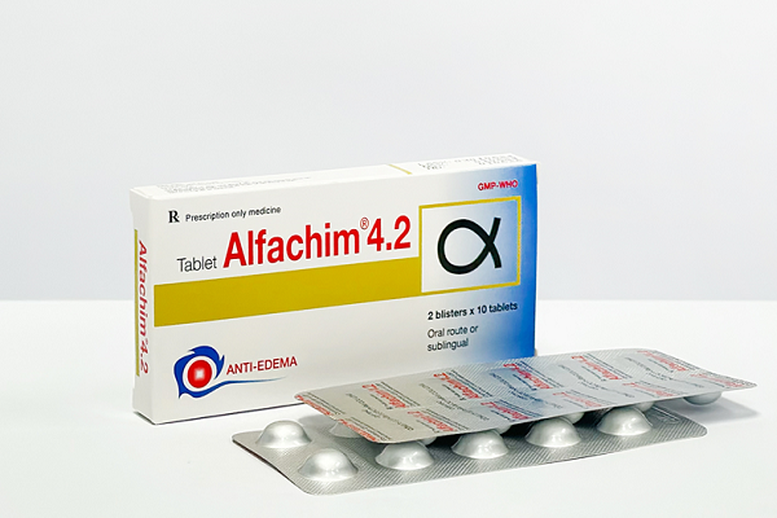
Alfachim 4.2 tablets are anti-edema and anti-inflammatory drugs, used in the treatment of edema after trauma, burns, and after surgery. This drug is very commonly used.
The recalled batch of medicine is labeled as Alfachim 4.2 tablets (Chymotrypsin (equivalent to 21 microkatal chymotrypsin) 4200 IU), circulation registration number: VD-34573-20, batch number 03010624; manufacturing date: June 1, 2024; expiry date: June 1, 2026, manufactured by Cuu Long Pharmaceutical Joint Stock Company.
According to the Drug Administration of Vietnam, in May 2025, the Central Institute for Drug Control tested the above drug sample, the results did not meet the quality standards for quantitative indicators (level 2 violation).
At that time, the Drug Administration requested the recall of this batch of medicine in Hanoi ; at the same time, it requested Cuu Long Pharmaceutical Joint Stock Company to coordinate in taking additional samples and sending the samples to the Central Drug Testing Institute or the Ho Chi Minh City Drug Testing Institute to check the quality of the quantitative indicators.
However, after the required deadline, the company still did not report the results of the additional sample quality test, only reported the production, distribution of the drug and the recall report. After that, the company sent a document to the Drug Administration, requesting a voluntary recall of this batch of drugs.
Therefore, the Drug Administration of Vietnam decided to announce a nationwide recall of this batch of drugs and requested the Company to coordinate with the distributor to promptly recall the entire batch of drugs that do not meet quality standards; send a recall report to the Department before July 8, 2025.
The Department also requested the Department of Health of provinces and cities, and the Department of Health of all sectors to notify drug trading and using establishments to recall the above substandard batch of drugs, publish information about the drug recall decision on the Department's website, inspect and supervise units implementing the notification; handle violators according to current regulations; report to the Department of Drug Administration and relevant authorities.
The Hanoi and Vinh Long Departments of Health are responsible for inspecting and supervising this company in recalling and handling the drug, and assessing the effectiveness of the recall, whether the product is still able to continue to be circulated and used, and whether it poses a risk of adversely affecting the health of users.
Suspending 20 types of cosmetics violating labeling and usage regulations
Recently, the Drug Administration of Vietnam, Ministry of Health has also suspended circulation and recalled 20 cosmetic products of Dai Cat A International Company Limited located at 43D/44 Ho Van Hue, Ward 9, Phu Nhuan District, Ho Chi Minh City, responsible for bringing them to the market.
The reason for the recall is that some products violate labeling regulations and their uses are not consistent with the approved declaration documents.
The Drug Administration of Vietnam also requested the Department of Health of provinces and cities to immediately stop trading and using violating products; request refunds to suppliers, and recall all violating products as well as monitor and handle violations in accordance with current regulations.
For Dai Cat A International Company Limited, the Drug Administration requires that a recall notice be sent to all distribution points, the products be recalled from business establishments and destroyed (if the offending label cannot be separated from the product) according to the provisions of Decree 126/2021/ND-CP.
Dai Cat A International Company Limited must report the recall results to the Drug Administration of Vietnam before June 30, 2025.
The Drug Administration of Vietnam requested the Ho Chi Minh City Department of Health to monitor the recall implementation and report the monitoring results to the Department before July 10, 2025.
Along with the above decision, the Drug Administration Department also revoked 20 receipt numbers of the cosmetic product declaration forms of Dai Cat A International Company Limited. At the same time, the Drug Administration Department temporarily stopped reviewing and receiving cosmetic product declaration dossiers for Dai Cat A International Company Limited for a period of 6 months, starting from June 20.
Hien Minh
Source: https://baochinhphu.vn/thu-hoi-lo-thuoc-pho-bien-dieu-tri-phu-ne-sau-chan-thuong-bong-102250623144940498.htm



![[Photo] Prime Minister Pham Minh Chinh chairs the national online conference on combating smuggling, production and trade of counterfeit goods.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/6/23/4a682a11bb5c47d5ba84d8c5037df029)
![[Photo] Prime Minister Pham Minh Chinh holds meeting to launch exhibition of national achievements to celebrate 80th National Day](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/6/23/0c0c37481bc64a9ab31b887dcff81e40)






















![[Photo] Party Congress of the Central Internal Affairs Commission for the 2025-2030 term](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/6/23/5bf03821e6dd461d9ba2fd0c9a08037b)





































































Comment (0)