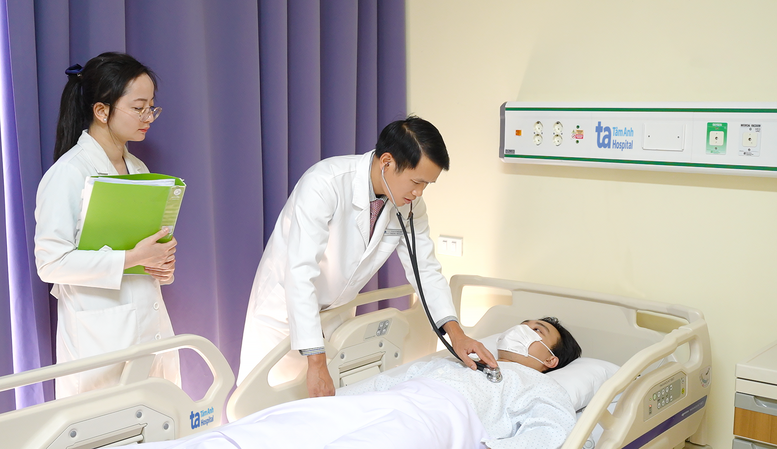
Patients participating in the VISTA-1 study receive comprehensive care at Tam Anh General Hospital.
Patients can access new drugs right in the country.
VISTA-1 study on oral immunotherapy drug RBS2418, developed by Riboscience (USA), with the participation of experts from Stanford University (USA). VISTA-1 was approved by the US Food and Drug Administration (FDA) in September 2024 and licensed by the Vietnamese Ministry of Health in December 2024.
In the phase 1 study in the US, RBS2418 recorded initial data on safety and tolerability. Phase 2A is the phase of evaluating the effectiveness of the invented drug. This is the first time Vietnam has participated in a clinical study of a cancer treatment drug right from phase 2A as the main research point.
Tam Anh General Hospital System is the first and only unit outside the US to participate in this research in the world . Currently, Tam Anh General Hospital has enough human resources and modern lab equipment to perform "central testing" right on the spot, without having to transfer test samples abroad as before.
Previously, Tam Anh General Hospital received and screened hundreds of patients, of which the first 8 qualified people are being treated according to the VISTA-1 research protocol.
Dr. Vu Huu Khiem, Head of the Oncology Department, Tam Anh General Hospital, Hanoi , the main researcher, said that the patients are being closely monitored for safety and response to the research drug. The research team has not recorded any significant abnormal symptoms related to the research drug in patients using the drug in the past 3 months.
" For patients with advanced colorectal cancer who no longer respond to current treatment regimens, the patient's survival time during the research process is very important. Currently, we and the patients have entered the fourth month of implementing this research," said Dr. Vu Huu Khiem.

The Bench Mark Ultra immunohistochemistry and fluorescence in situ hybridization analyzer from the US helps detect and distinguish many types of cancer.
According to Dr. Phuong Le Tri, Executive Director of Tam Anh Research Institute (TAMRI), in the past, Vietnamese patients, especially those with serious illnesses such as cancer, often had limited information about new and advanced drug research protocols in the world, so they had to seek opportunities abroad.
Vietnam’s participation in international studies such as VISTA-1 allows patients to access new drug candidates right at domestic hospitals, instead of having to go abroad. Vietnamese scientists and doctors also have the opportunity to access professional research processes and world-leading innovative drugs early, instead of only accessing them at a late stage in previous research.
World-class lab system for potential cancer drug research
According to Dr. Vu Huu Khiem, the initial positive feedback from patients is an important observation in the monitoring process, supporting the assessment of the safety and effectiveness of the drug RBS2418. Patients will continue to be monitored and evaluated at the expected time points of weeks 6, 12 and 20, through computed tomography scans, biological indicators and quality of life assessment questionnaires.
Prof. Dr. Jeffrey S. Glenn, Director of the Stanford Institute for Microbiology and Infectious Diseases (USA), head of the VISTA-1 research team, said he was very impressed with the progress of patient recruitment in Vietnam over the past 3 months.
" We appreciate the patient selection and management process, as well as the quality of the laboratory and research infrastructure of Tam Anh General Hospital, especially the effective coordination between the research teams in the US and Vietnam, " Professor Jeffrey emphasized.
VISTA-1 is the first international study in the field of cancer that Vietnam has participated in since phase 2A. To prepare for this important study, Tam Anh General Hospital System and Tam Anh Research Institute (TAMRI) have invested heavily to develop a central laboratory that meets ISO 15189 standards for the fields of Hematology - Biochemistry - Microbiology and Molecular Biology of the Ministry of Health.
The Lab is equipped with many modern specialized devices for research such as Parsortix technology to support the separation and collection of circulating tumor cells in peripheral blood (CTC) for research; Leica Bond RX automatic immunohistochemistry staining system to evaluate the expression level of two biomarkers ENPP1 and cGAS in cancer cells and tissues.
This is the first time these technologies have been deployed in the clinical research system in Vietnam. Tam Anh General Hospital is also the first unit in Vietnam to be transferred the testing techniques for two new biomarkers ENPP1 and cGAS, thereby qualifying to participate in the VISTA-1 study.
The VISTA-1 study will continue to recruit patients in Vietnam and the US until the required number is reached. Patients participating will be regularly checked for their health and disease progression; at the same time, they will be supported with costs related to research procedures, travel expenses, and have all questions answered 24/7 by the research team of experts.
HM
Source: https://baochinhphu.vn/8-benh-nhan-dau-tien-thu-nghiem-thuoc-moi-dieu-tri-ung-thu-cua-my-102250506120716036.htm



![[Photo] Anh Hoang - Dinh Duc successfully defended the men's doubles championship of the National Table Tennis Championship of Nhan Dan Newspaper](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/23/d6ab3bcac02c49928b38c729d795cac6)















![[Photo] Top players gather at the 2025 Nhan Dan Newspaper National Table Tennis Championship](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/23/9ad5f6f4faf146b08335e5c446edb107)






























![[Photo] The Central Party Executive Committee delegation visits former President Tran Duc Luong](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/24/32f67673454445aab0f1f2af331cb170)



































Comment (0)