Super clean environment
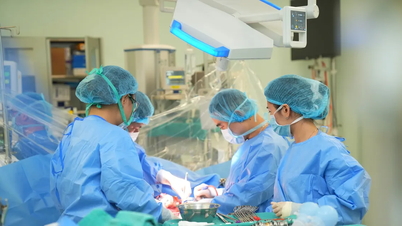
According to AP , in the middle of the Blue Ridge Mountains in Virginia, a pig farm that looks like a pharmaceutical factory operates quietly.
Here, every employee entering the breeding area must shower, change clothes, disinfect shoes, and follow procedures more strict than those in a hospital. Every sip of water, every breath of the pigs is filtered through many layers of safety, and food is also disinfected before being put into the pen.
Facility in Christiansburg, Virginia, USA (Photo: UTC).
Inside the facility, Revivicor, a subsidiary of United Therapeutics, is growing a herd of pigs, each treated as a “living medical device,” with the goal of providing biocompatible kidneys and hearts.
The goal is not only to raise healthy pigs, but also to produce clean, safe organs that are free of pathogens that can be transmitted to humans.
A few miles away in Christiansburg, another $75 million facility is expanding, the first in the United States to raise pigs in a pathogen-free environment, with full-fledged transplants expected to begin in 2026.
In a space of more than 7,000 square meters, there is a multi-layered air filtration system, backup drinking water is stored in giant tanks and the breeding area is completely isolated.
Here, the piglets are separated from their mothers after 1 or 2 days, transferred to super clean pens and cared for entirely by hand. Staff must change masks and protective gear when entering each pen, ensuring absolutely no cross-contamination of germs.
Not only living in ideal conditions, the pigs also listen to music, play ball and interact with people to get used to sounds and a living environment similar to humans.
From gene editing to the hope of replacing human organs
The shortage of organs for transplant in the US has been going on for decades. Every year, thousands of patients die because they cannot find suitable donors. Revivicor began to solve this problem by altering the genes of pigs to make them more compatible with the human body.
David Ayares, president and chief scientific officer of Revivicor, looks at pigs at the company's research farm (Photo: AP).
Scientists selected pig skin cells and removed immune-reactive genes such as the gene that produces alpha-gal, which causes near-instant rejection.
They then removed three more genes to remove the signals that activate the immune system. They also inserted human genes to reduce the risk of blood clots and adjusted the size of the organ to match the recipient.
The process takes place like a complex jigsaw puzzle in the laboratory.
After implanting new genetic material into pig eggs with an electric shock, the embryos are grown in a hand-held incubator and then implanted into pregnant sows. From there, the first cloned pigs are born, carrying genes pre-programmed to grow replacement organs for humans.
Revivicor’s first gene-edited pig was named GalSafe. After initial success, the company began breeding the pig instead of continuing with mass cloning. The scientific team hopes to move toward producing hundreds of organs a year for large-scale transplants.
Pig-to-human transplants are no longer a distant dream.
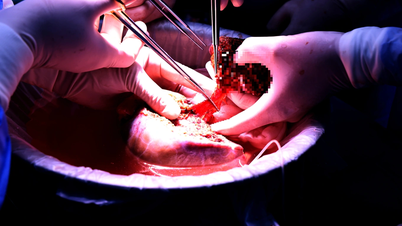
Four pig-to-human organ transplants have been performed in the United States, including two heart transplants and two kidney transplants. Although the patients died within a few months, each case provided valuable data, opening up hope for future transplants on less ill patients.
Current trials continue on baboons – primates that are biologically close to humans – to assess the safety of pig organs before they are officially approved by the FDA.
Pigs are raised in super clean conditions (Photo: UTC).
Scientists believe that, thanks to gene editing techniques and sterile farming conditions, organs from pigs will avoid rejection reactions and reduce the risk of potential disease transmission.
About 300 pigs of various ages now live in Revivicor’s farm system, each with an identification tag and a detailed genetic profile. A small group will be retained for the most important tests, including the first human clinical trials in the near future.
Professor David Ayares, the geneticist leading the project, said if further trials were successful, the company would continue to expand its production facilities, aiming to produce 2,000 organs a year.
Source: https://dantri.com.vn/khoa-hoc/bi-mat-tai-noi-nuoi-loai-lon-sach-nhat-the-gioi-de-ghep-tang-cho-nguoi-20250729081634461.htm







































































































Comment (0)