
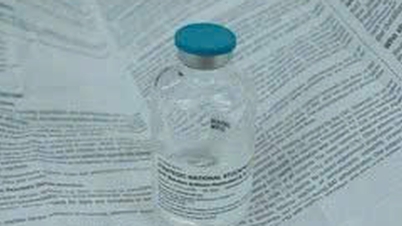
Product of DOCTOR MAGIC brand. (Photo: Screenshot)
According to the results of data lookup on the Online Public Service System for Receiving Cosmetic Declarations connected to the National Single Window Portal, from 2018 to present, the Drug Administration of Vietnam has received 162 Cosmetic Product Declaration Forms from this company; as of November 17, there are still 81 valid receipt numbers.
The Drug Administration of Vietnam has documents requesting the health departments of provinces and cities to provide information on inspection, examination and handling of administrative violations (if any) against MK Skincare Company; requesting the health departments of provinces and cities, the Central Institute for Drug Testing, and the Ho Chi Minh City Institute for Drug Testing to take samples and test the quality of the above-mentioned cosmetic products of MK Skincare Company, requesting the company to provide information on test results and product information records.
While the test results of the units are not yet available, the Drug Administration recommends that businesses and people need to be careful in using cosmetic products imported and announced by MK Skincare Company.
Source: https://nhandan.vn/than-trong-khi-su-dung-my-pham-do-cong-ty-mk-skincare-nhap-khau-cong-bo-post924099.html




![[Photo] President Luong Cuong receives President of the Senate of the Czech Republic Milos Vystrcil](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F20%2F1763629737266_ndo_br_1-jpg.webp&w=3840&q=75)
![[Photo] National Assembly Chairman Tran Thanh Man holds talks with South Korean National Assembly Chairman Woo Won Shik](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F20%2F1763629724919_hq-5175-jpg.webp&w=3840&q=75)

![[Photo] Lam Dong: Panoramic view of Lien Khuong waterfall rolling like never before](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F20%2F1763633331783_lk7-jpg.webp&w=3840&q=75)





























































































Comment (0)