The Drug Administration of Vietnam ( Ministry of Health ) said it had received a report from the Hanoi Center for Drug, Cosmetic and Food Testing about 7 drug samples without information on the circulation registration number or import license number, information on the drug manufacturing facility, and drug importing facility.
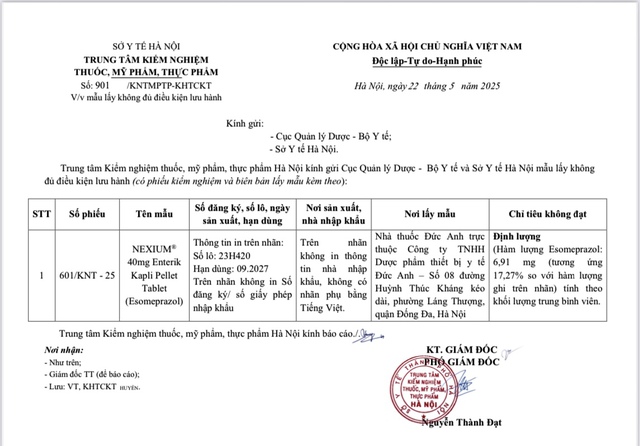
Nexium sample among 7 product samples at Duc Anh pharmacy is not eligible for circulation
PHOTO: DEPARTMENT OF DRUG ADMINISTRATION
These samples were taken at Duc Anh pharmacy, belonging to Duc Anh Pharmaceutical and Medical Equipment Company Limited (address: 8 Huynh Thuc Khang extended street, Dong Da district, Hanoi).
In which, the product sample Diamicron ® MR 60mg (gliclazide), batch number 23F603, expiry date 4.2026 did not meet the quality requirements for gliclazide quantification (70.83% compared to the content stated on the label).
The other 6 products are:
Oseltamivir, lot number M1164B01; manufacture date 3.2021, expiry date 3.2023.
Crestor 20mg (rosuvastatin); lot number A23237030, expiry date 4.2026.
Janumet 50/1000mg (sitagliptin/metformin); lot number 24497505A, expiry date 7.2026
Plavix (klopidogrel); lot number ELB04027, expiry date 5.2027.
Nexium ® 40mg Enterik Kapli pellet tablet (esomeprazole), batch number 23H420, expiry date 9.2027.
Crestor 10mg (rosuvastatin); lot number A24236004, expiry date 7.2027.
The Drug Administration of Vietnam requested the Hanoi Department of Health to coordinate with relevant agencies to inspect and examine the compliance with pharmaceutical laws of Duc Anh Pharmacy, and at the same time trace the origin of 7 batches of products without information on the circulation registration number, import license, manufacturing facility, and importing facility.
The Drug Administration has notified health departments nationwide, requesting information to drug trading and usage establishments and people to know not to buy, sell, or use the 7 batches of products mentioned above; instructing users to only buy drugs at legal pharmaceutical businesses. Distribution establishments are not allowed to buy or sell drugs of unknown origin.
According to information from the treatment facility, the 7 products mentioned above are widely used drugs for diabetes; antiviral and flu prevention; treatment of hypercholesterolemia; prevention of blood clots and cardiovascular events; treatment of diseases such as gastroesophageal reflux...
Source: https://thanhnien.vn/ha-noi-phat-hien-7-mau-thuoc-khong-ro-nguon-goc-185250530060135785.htm



























































































Comment (0)