Accordingly, in Decision No. 628/QD-QLD dated October 31 issued by the Department of Drug Administration, Ministry of Health , 14 vaccines and biological products were granted a registration certificate for circulation in Vietnam with a validity of 3 years.
Among them, there is the drug Pembroria (main active ingredient is Pembrolizumab, content 100mg/4ml) produced by Limited Liability Company "PK-137" (Russia), a facility registered in the United Arab Emirates.
Pembroria medicine is prepared in the form of a concentrated solution for infusion, with a shelf life of 24 months from the date of manufacture.
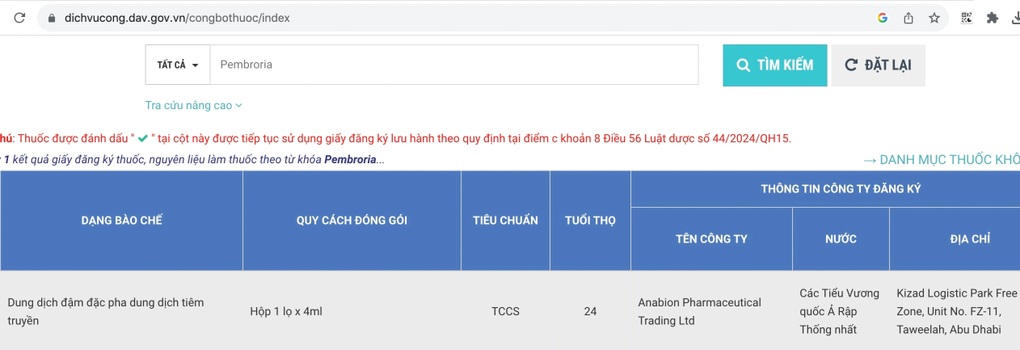
The product is announced and licensed on the online public service portal of the Drug Administration of Vietnam, Ministry of Health , valid until October 31, 2028 (Screenshot).
The leader of the Drug Administration Department said that information from the Drug and Biological Products Registration Office showed that the reference biological product had been granted a circulation registration certificate for many years. This Pembroria drug is produced by Russia, is a similar biological product, and has just been granted a circulation registration certificate.
"The product is licensed for widespread circulation in Vietnam, not for clinical trials. The issuance of a circulation registration certificate allows the drug to be imported, distributed and widely used like other drugs, because it does not belong to a special or restricted group of drugs," said the leader of the Drug Administration Department.
Previously, many drugs with effects and indications like Pembroria were granted registration for circulation in Vietnam.
Associate Professor, Dr. Pham Cam Phuong, Director of the Center for Nuclear Medicine and Oncology, Bach Mai Hospital, said that targeted drugs for cancer treatment have been available in Vietnam for many years. The new Russian drug that has just been licensed in Vietnam is a similar biological product.
According to this expert, adding one more drug will help more patients have access to it at a reasonable price.
Sharing this view, Professor Dr. Le Van Quang, Director of K Hospital, said that K Hospital will soon introduce this drug into treatment for patients.
The price of Pembroria medicine produced by Limited Liability Company "PK-137" (Russia) is about 18 million VND/bottle. Patients usually use 2 bottles for 1 treatment course.
Patients will be given 12-24 courses of medication until they no longer respond to the medication.
However, this new drug is not yet covered by health insurance.
Source: https://dantri.com.vn/suc-khoe/thuoc-chua-ung-thu-cua-nga-vua-duoc-cap-phep-co-gia-18-trieu-mot-lo-20251112124324919.htm


![[Photo] The "scars" of Da Nang's mountains and forests after storms and floods](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1762996564834_sl8-jpg.webp)

![[Photo] General Secretary To Lam visits Long Thanh International Airport Project](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1763008564398_vna-potal-tong-bi-thu-to-lam-tham-du-an-cang-hang-khong-quoc-te-long-thanh-8404600-1261-jpg.webp)



















































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)








Comment (0)