Hot weather and power outages can affect vaccine quality, so vaccination facilities must always have backup plans to ensure storage.
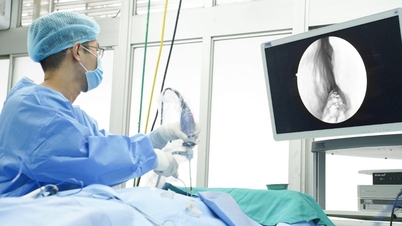
According to Ms. Ngo Thi Tuyet Suong, Quality Director of VNVC Vaccination System, vaccines are special biological products used on humans, directly introducing antigens into the body to create active immunity. Most vaccines need to be stored and transported at a temperature of 2-8 degrees Celsius with a cold storage system, refrigerator, and specialized refrigerated vehicles.
Some vaccines are sensitive to low temperatures: such as hepatitis B; diphtheria-tetanus-pertussis (DTP); tetanus-diphtheria (TD); tetanus, typhoid, can be damaged when frozen. Some other vaccines are sensitive to high temperatures such as oral polio (OPV); measles-rubella (MR); measles-mumps-rubella (MMR) can be damaged when exposed to high temperatures or light.
Ms. Suong explained that high temperatures can cause the main components of the vaccine to change or lose their properties. This can reduce the effectiveness of the vaccine or create by-products that can be harmful. In addition, high temperatures can affect the stability of the vaccine, leading to a decrease in the quality and effectiveness of the vaccine. When the vaccine is damaged, its protective effect is reduced or lost, and the vaccinated person may experience complications. Therefore, storing the vaccine at the right temperature is an important condition to ensure the safety and effectiveness of vaccination.
Therefore, vaccination centers must have a backup plan to ensure absolute quality of vaccines and avoid risks to vaccinated people. At VNVC, backup plans for vaccine preservation are strictly implemented at all stages.
Each cold storage of VNVC always has at least 2 cooling units and operates alternately. In terms of capacity, one cooling unit is enough for the cold storage to operate at a temperature of 2-8 degrees Celsius, the arrangement of two cooling units is for backup in case one of the two cooling units has a problem.
To avoid the risk of power outages affecting vaccine quality, vaccination centers always ensure there are two power sources for the cold chain (including the national grid and generators). When there is a power grid problem, the generator will operate to ensure power supply for cold storage and refrigerators. VNVC is equipped with large capacity generators to ensure continuous power supply for 72 hours. Every month, the generators will be tested with no load and load to ensure that when there is a power grid failure, the generators are ready and in good working condition.

Vaccine cold storage meets GSP standards at VNVC. Photo: Moc Thao
To keep vaccines at 2-8 degrees Celsius, VNVC has a strict and diverse monitoring and warning system with many warning channels. When the temperature is outside the threshold (below 3 degrees and above 7 degrees), the warning system will be activated, helping the monitoring team detect early and take timely measures, ensuring that the vaccines are always stored as required. The system of equipment for monitoring storage temperature, automatic temperature monitoring 24/24 hours on-site and online includes 3 layers: on-site warning with siren and light signals, remote warning via SMS and warning via email to responsible people such as warehouse keepers, warehouse managers, quality managers and maintenance... if the temperature is at a dangerous threshold.
At VNVC, vaccines are stored in a cold storage system that meets international Good Storage Practices (GSP) standards and a closed cold storage chain system. With a network of 4 main warehouses and nearly 115 cold storages located at vaccination centers nationwide, VNVC can store nearly 300 million doses of vaccine at the same time, including a storage system that can reach minus 86 degrees.
Mr. Nguyen Huu Hanh, Logistics Director of VNVC, said that the system is also ready to mobilize mobile generators within one hour of the request. This is to ensure timely power supply in case of problems with both of the above power sources. In addition, VNVC also prepares plans to mobilize mobile cold storage from neighboring centers. All employees such as warehouse staff, maintenance, quality assurance, even warehouse security, vaccine transport drivers, are regularly trained to handle cold storage problems.
"In principle, if there is a power outage, the cold storage can still ensure that the vaccine is preserved at a temperature of 2 to 8 degrees Celsius for 60 minutes, while it only takes about 20 seconds for the generator to automatically operate," said Mr. Hanh.
Refrigerated trucks, cold boxes, cold storage tanks, and gel ice are prepared to package vaccines when needed. All cold boxes are equipped with self-recording thermometers, devices considered as "black boxes" of vaccines, to retrieve temperature data throughout the journey and monitor temperature levels in real time. The temperature of the cold box is continuously displayed in the cockpit. This is also the basis for VNVC to evaluate the quality of vaccines during transportation and warehousing.
Chile
As a comprehensive strategic partner of many vaccine companies in the world such as Glaxosmithkline - GSK (Belgium), Sanofi Pasteur (France), Pfizer (USA), Merck Sharp and Dohme (USA), AstraZeneca (UK), VNVC imports genuine vaccines or pre-orders large quantities of vaccines. Among them are many scarce vaccines such as vaccines against tuberculosis, rotavirus, pneumococcus, seasonal flu, meningitis caused by meningococcus, Gardasil/Gardasil 9 against HPV virus... In addition, the system also has products with the same function as expanded immunization vaccines such as 6 in 1 (preventing diphtheria, whooping cough, tetanus, hepatitis B, pneumonia/meningitis caused by Hib bacteria, polio), measles - mumps - rubella, tuberculosis vaccine, whooping cough - diphtheria - tetanus.
Source link


![[Photo] Cat Ba - Green island paradise](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F04%2F1764821844074_ndo_br_1-dcbthienduongxanh638-jpg.webp&w=3840&q=75)




















































![[VIMC 40 days of lightning speed] Hai Phong Port determined to break through, reaching the target of 2 million TEUs by 2025](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/12/04/1764816441820_chp_4-12-25.jpeg)





















































Comment (0)