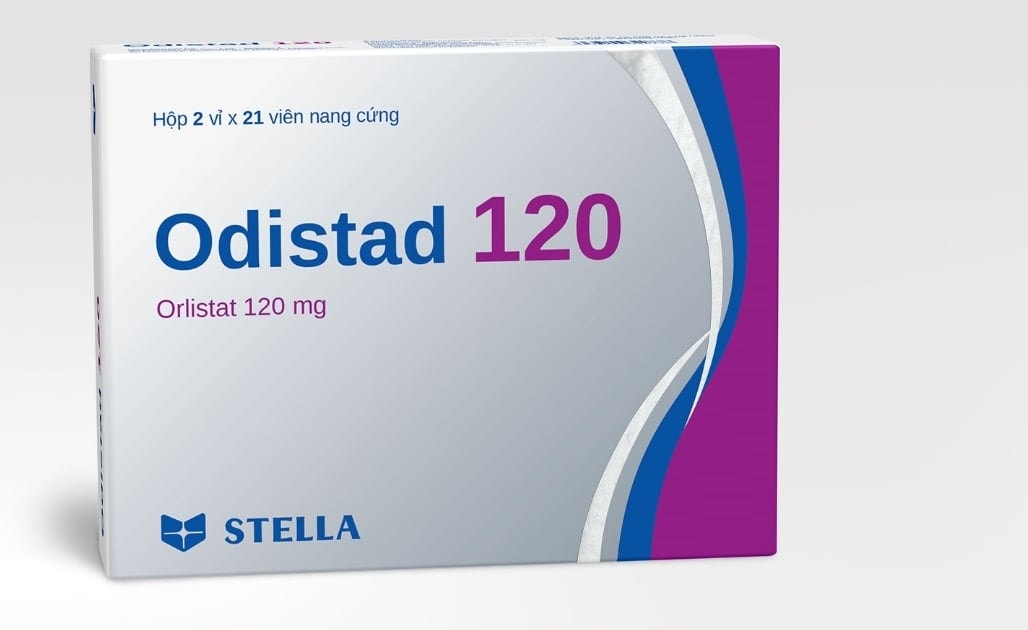
The Drug Administration of Vietnam ( Ministry of Health ) issued a decision to revoke the circulation registration in Vietnam for three drugs to treat erectile dysfunction, obesity, and pain and fever reduction.
The recalled drugs include:
- Tadalafil 20mg, a prescription drug for treating erectile dysfunction in men, is registered and manufactured by Meyer-BPC Joint Venture Company ( Ben Tre province).
- Odistad 120, a drug to support the treatment of obesity and prevent weight gain, is registered by Stellapharm Joint Venture Company Limited ( Ho Chi Minh City) and manufactured at its branch in Binh Duong.
- Vacobufen 400 - Ibuprofen 400mg, pain reliever, antipyretic, anti-inflammatory, registered and manufactured by Vacopharm Pharmaceutical Joint Stock Company (Long An).
The reason for the recall is that the businesses voluntarily requested to withdraw their registration certificates. According to the decision, drugs manufactured before the recall date are still allowed to be circulated until their expiry date. The registration and production facilities are responsible for ensuring the quality, safety and effectiveness of the drugs during the circulation process.
The Drug Administration of Vietnam requests the Directors of the Departments of Health of provinces and cities, along with relevant units, to coordinate in implementing the decision.
This move comes as authorities have recently discovered cases of counterfeit drugs, fake milk, low-quality functional foods and cosmetics. Many artists have also been criticized for false advertising and exaggerating product benefits.
On May 22, the Ministry of Health established 15 surprise inspection teams to simultaneously inspect pharmaceuticals, cosmetics, traditional medicine, milk, health food and medical equipment.
PVSource: https://baohaiduong.vn/thu-hoi-giay-dang-ky-luu-hanh-loat-thuoc-tri-roi-loan-cuong-beo-phi-giam-dau-413181.html



































































































Comment (0)