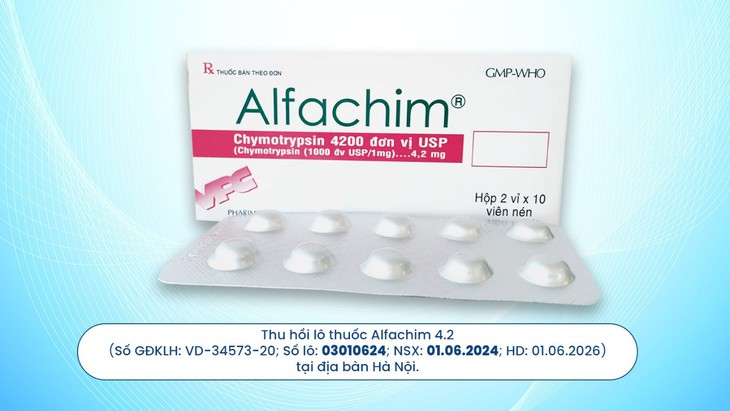
Accordingly, based on Official Dispatch 1377/QLD-CL of the Department of Drug Administration - Ministry of Health dated May 20, 2025 regarding the drug sample Alfachim 4.2, registration number VD-34573-20; batch number 03010624; production date: June 1, 2024; expiry date June 1, 2026, manufactured by Cuu Long Pharmaceutical Joint Stock Company does not meet quality standards for quantitative indicators for circulation in Hanoi.
Cuu Long Pharmaceutical Joint Stock Company said that on May 21, 2025, immediately after receiving instructions from the Department of Drug Administration, the Company immediately issued Official Letter No. 1086/CV.DCL notifying the recall of Alfachim 4.2 product, registration number: VD-34573-20; batch number: 03010624; production date: June 1, 2024; expiry date: June 1, 2026 to distribution partners in Hanoi and establishments selling and using drugs provided by Ngoc Khanh Pharmaceutical - Medical Supplies Joint Stock Company (Counter 312A, 3rd floor, Hapu Center - No. 1, Nguyen Huy Tuong, Thanh Xuan, Hanoi).
The notice stated the recovery process and the deadline for completing this work is June 2, 2025 throughout Hanoi .
The recall will be strictly carried out by Cuu Long Pharmaceutical Joint Stock Company and its branch in Hanoi in accordance with the instructions of the Drug Administration Department as well as current legal regulations.
At the same time, Cuu Long Pharmaceutical Joint Stock Company has urgently reviewed the entire production process, quality control and product distribution.
On May 26, 2025, Cuu Long Pharmaceutical also sent Official Dispatch 244/CV.DCL to the Department of Drug Administration - Ministry of Health; Central Institute for Drug Testing; Ho Chi Minh City Institute for Drug Testing; Vinh Long Provincial Department of Health to report in detail on the production situation (production quantity, supply quantity, expiry date, production date, registration number, batch number, active ingredient name) and the distribution situation of the drug batch (name and address of the business establishment that purchased the drug, quantity purchased and in stock).
Currently, Cuu Long Pharmaceutical said it is working closely with the authorities to find out the cause of the incident. First of all, it is coordinating to collect additional samples at business establishments to check the quality of the quantitative indicators of these drug samples, thereby having a basis to assess the subjective and objective causes that may affect product quality.
"Cuu Long Pharmaceutical Joint Stock Company commits to fully cooperate with the authorities and is ready to take responsibility according to regulations if the cause is determined to originate from the company's production or quality control process," the company said.
While waiting for the official conclusion from the authorities, Cuu Long Pharmaceutical Joint Stock Company continues to conduct a comprehensive review of the quality management system, especially in the production and inspection stages, considering this an opportunity to further consolidate and improve quality standards - the foundation that has built the reputation of Cuu Long Pharmaceutical over the past 50 years.
Cuu Long Pharmaceutical representative said that the company understands that reputation is an invaluable asset of a business, especially in the pharmaceutical and medical field. And that reputation must be built on product quality, transparency, and accuracy in system management.
Therefore, the company is committed to acting responsibly, receptively and decisively to be worthy of the trust of customers, partners and the whole society.
With nearly 50 years of operation in the pharmaceutical field, Cuu Long Pharmaceutical Joint Stock Company has become a familiar name to consumers, pharmacies and major hospitals in provinces and cities of Vietnam and is committed to providing quality, safe and effective products.
With the efforts achieved, in recent years, the company has been honored to receive many prestigious awards such as: Second Class Labor Medal;
Top 10 Consumer Trusted Products and Services; "Vietnamese Medicine Star" Award; Top 10 Famous Brands; Top 10 Leading Brands in Vietnam 2022; Top 10 Leading Prestigious Brands in Asia and many other prestigious awards.
Source: https://tuoitre.vn/duoc-cuu-long-thong-bao-thu-hoi-san-pham-alfachim-4-2-so-lo-03010624-tai-ha-noi-2025053020103279.htm



























































































Comment (0)