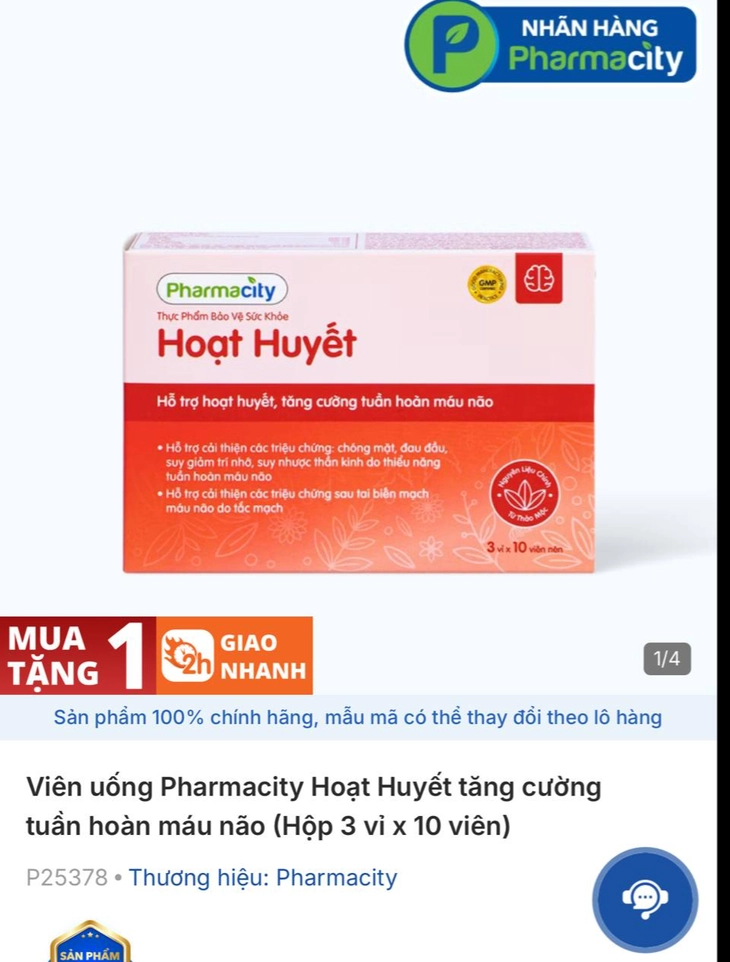
Pharmacity Blood Circulation Enhancement Pills manufactured by Herbitech Company
Confused about buying products produced by the company being prosecuted
As Tuoi Tre Online reported earlier, on April 28, the Institute of Criminal Science of the Ministry of Public Security determined that two health protection food products Medi Kid Calcium K2 and An ngon BABY SHARK produced by Herbitech Technology Company Limited were counterfeit.
The Ministry of Public Security's Investigation Police Agency has continued to seize 115 more samples of health protection products produced by Herbitech Technology Company Limited with signs of counterfeit goods, awaiting appraisal results for the above product samples.
Sharing with Tuoi Tre Online , Mr. C. said that for over a year he has regularly bought Pharmacity Ginkgo Biloba tablets to support blood circulation and increase brain circulation (box of 30 tablets); Pharmacity Blood Circulation Enhancement tablets to enhance brain blood circulation (box of 3 blisters x 10 tablets) and Pharmacity Joint Care (30 tablets), a type that helps reduce symptoms of joint pain and dry joints due to arthritis at Pharmacity.
"Previously, when buying functional foods, I only cared about the ingredients, dosage and expiry date. However, after a series of fake drug busts, I became interested in information related to the manufacturer.
After checking carefully, I discovered that the products I bought were from Herbitech Technology Company Limited - a company that was recently prosecuted for producing counterfeit goods," said Mr. C.
Although these products have not been announced as counterfeits yet, the fact that the products were produced by a company that was prosecuted makes Mr. C. very confused.
As soon as he discovered it, on May 8, he called Pharmacity's hotline to report it. The staff received the information and invited him to the store for an explanation, promising to provide evidence that the product met standards.
However, according to Mr. C., when he arrived at the store, the staff struggled to find the documents but could not present the relevant documents and promised to send them via Zalo. Up to now, after 3 times of contacting the hotline, he has not received a clear response.
According to research, the products Mr. C. bought have the following information: Announced unit: Hoang Giang Pharmaceutical Company Limited; manufacturer: Noi Bai Factory - Herbitech Technology Company Limited and the distribution unit is Pharmacity Pharmaceutical Joint Stock Company.
Recall of products manufactured by Herbitech Company
Regarding the above reflection, on May 13, Pharmacity pharmacy said it had officially announced to proactively recall a number of functional products manufactured by Herbitech Company.
Speaking to Tuoi Tre Online , Ms. Do Thi Thuy Van, communications director of Pharmacity, said that after receiving recommendations from authorities related to two products of Herbitech Technology Company Limited (Herbitech Company), Baby Shark and Medi Kid Calcium K2, Pharmacity pharmacy recalled all products related to Herbitech Company and its affiliated companies.
In addition, Pharmacity pharmacy has proactively suspended business and recalled products manufactured by Herbitech Company, including 4 products: PMC Hoat Huyet; PMC Gingko Biloba; PMC Joint Care and PMC Liver Support.
These products are registered, announced and distributed by Hoang Giang Saigon Pharmaceutical Company Limited.
Ms. Van explained that before and during the distribution of these products, Pharmacity pharmacy proactively spent money to conduct periodic product quality checks at the VNTest Quality Control and Testing Institute.
The Company also collected reports provided by Hoang Giang Saigon Pharmaceutical Company Limited, on behalf of Herbitech Company.
"Each report, including the independent inspection report and the inspection report provided by Hoang Giang Saigon Pharmaceutical Company Limited to Pharmacity pharmacy, shows that the quality of all four products meets the standards according to the published records licensed by the Department of Food Safety ( Ministry of Health ).
Although there has been no official conclusion from the authorities, the suspension of business is still being carried out by Pharmacity Pharmacy with the highest goal of ensuring the health and safety of consumers, while demonstrating Pharmacity Pharmacy's commitment to strictly complying with legal regulations on product quality standards," Ms. Van informed.
In addition, to ensure customer peace of mind, Pharmacity has proactively taken responsibility for recalling and refunding 100% to all customers who have purchased products related to Herbitech Technology Company Limited.
"Customers who have purchased the above products can bring the products to any Pharmacity pharmacy nationwide to receive a quick and convenient refund," said Ms. Van.
Although information from Pharmacity stated that the products were recalled after the authorities prosecuted Herbitech Company (on April 28, the company was prosecuted). However, on May 8, these products were still being sold publicly.
After receiving customer feedback, on May 10, the above products were no longer displayed on Pharmacity's official website.
On May 12, in Hanoi , when I went directly to the store to buy these products, the staff said that both Pharmacity Hoat Huyet and Pharmacity Joint Care products were "out of stock", with no clear time of availability. The staff here did not provide any instructions on how to return the products.
It was not until May 13 that the company had official written information about the product recall.
Mr. C. said that he has not received any official response from the pharmacy chain regarding the quality of the products that his relatives have used over the past year.
Source: https://tuoitre.vn/hoang-mang-vi-mua-san-pham-do-cong-ty-co-hang-gia-san-xuat-ban-tai-nha-thuoc-20250513083528642.htm



![[Photo] General Secretary To Lam receives Chief of the Central Office of the Lao People's Revolutionary Party](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/30/140435f4b39d4599a3d17975dfb444c5)









![[Video] Ho Chi Minh City: Number of COVID-19 cases increases rapidly, 2 deaths recorded](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/31/5fe289cf72774d918f23fe88bb1686ce)












![[Photo] National Conference "100 years of Vietnamese Revolutionary Press accompanying the glorious cause of the Party and the nation"](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/30/1cf6cd5c8a934ebfa347028dcb08358c)
































































Comment (0)